Abstract
Antiphospholipid antibodies including anticardiolipin antibodies, lupus anticoagulants, and anti–β2 glycoprotein-1–specific antibodies may identify patients at elevated risk of first or recurrent venous or arterial thromboembolism. Traditionally, published case series supplemented by anecdotal experience have formed the basis of management of patients with these autoantibodies. Over the past several years, studies have described the management of patients with key clinical manifestations of antiphospholipid antibodies, including patients with antiphospholipid antibody syndrome. As a result, evidence-based treatment recommendations are possible for selected patients with, or at risk of, thrombosis in the setting of antiphospholipid antibodies. Unfortunately, most patients encountered in clinical practice do not correspond directly with those enrolled in clinical trials. For such patients, treatment recommendations are based on experience, extrapolation, and less rigorous evidence. This article proposes 5 cases typical of those found in clinical practice and provides recommendations for therapy focused on a series of clinical questions. Whenever possible, the recommendations are based on evidence; however, in many cases, insufficient evidence exists, so the recommendation is experiential.
Introduction
Antiphospholipid syndrome (APS) is a prothrombotic disorder predisposing to thrombocytopenia, recurrent pregnancy morbidity, and thrombosis. This review will focus on the management of selected patients with, or at risk of, thromboembolism in the setting of known, or suspected, antiphospholipid antibodies (aPLs), including patients meeting consensus conference criteria for APS.
Although there remain many unanswered questions around the diagnosis and management of such patients, recent evidence does provide some guidance for their management. To avoid duplicating recent reviews,1-3 we present our opinions in a series of cases that illustrate typical clinical presentations. For clinical questions about what evidence is lacking, we offer our opinion about diagnosis or treatment options. We have, throughout the text, tried to clarify whether the strategy we endorse is supported by published evidence or is experientially based.
Establishing the diagnosis of APS
Case 1
A 25-year-old nulliparous woman presents with an objectively confirmed left leg proximal, deep vein thrombosis that occurred without precipitant.
Question: What laboratory testing should be ordered and when should the testing be performed?
Because of her young age and her unprovoked deep vein thrombosis (DVT), the possibility that this patient has an acquired or inherited “thrombophilia” must be considered. If laboratory testing were to reveal the presence of aPLs, it is quite possible that this patient has APS. Because patients with APS and a first episode of venous thromboembolism (VTE) have a high risk for recurrent VTE after anticoagulants are discontinued,2,4-6 laboratory testing for APS is indicated because a persistent positive test would mandate extended duration anticoagulation, as suggested in current consensus documents.7
The diagnosis of APS can be made only when a characteristic clinical presentation is combined with objective laboratory abnormalities that are present on 2 or more occasions at least 12 weeks apart (Table 1). Laboratory testing requires a positive test for at least one of the following: anticardiolipin antibodies (aCLs), anti–β2 glycoprotein-1 (β2 GP1) antibodies, or a lupus anticoagulant (LAC). Results of aCL and β2 GP1 antibody testing must be interpreted with the knowledge that many commercial and locally developed assays suffer from both a lack of standardization and significant batch-to-batch variability10,11 ; we suggest that clinicians familiarize themselves with the performance of the specific assay(s) used by their laboratory. It is our opinion that aCL levels (especially the IgG isotype) that are higher than 40 IgG phospholipid units (GPL) or IgM phospholipid units (MPI; or are > 99th percentile), if confirmed when repeated more than 12 weeks later, suggest that a patient with objectively documented thrombosis has APS. In contrast to aCL, testing for the LAC appears to be associated both with greater interlaboratory reproducibility12 and with a higher specificity for APS.11
At the time of her presentation with DVT, laboratory testing is positive for LAC. aCL levels (IgG and IgM) are within normal limits.
Question: Based on these results, what is the optimal type and intensity of anticoagulant therapy?
Like any patient with acute VTE who does not have a contraindication (such as a high risk of bleeding or prior history of heparin-induced thrombocytopenia), this patient should be started on therapeutic doses of a rapidly acting parenteral anticoagulant (eg, unfractionated heparin, a low-molecular-weight heparin [LMWH], or pentasaccharide) and an oral vitamin K antagonist such as warfarin.7 After at least 4 to 5 days of “overlap,” and assuming a stable international normalized ratio (INR) between 2.0 and 3.0, the patient can be treated with a vitamin K antagonist alone. The recommendation to use warfarin administered with an INR of 2.0 to 3.0 is based on the results of 2 randomized, controlled trials that enrolled patients with APS; both found that treatment to achieve this INR effectively reduced the risk of recurrent venous thrombosis.13,14
Question: How long should vitamin K antagonist treatment be continued?
If repeat aPL testing (performed at least 12 weeks after the initial samples were collected) demonstrates persistent evidence of a LAC or elevated levels of aCL/β2 GP1 antibodies, it is reasonable to conclude that this patient has APS; however, it should be remembered that diagnostic assays for LAC may be falsely positive in some warfarin-treated patients. Thus, clinicians should consider interrupting warfarin therapy for a brief period (several days) prior to obtaining a plasma sample for LAC testing.
Prospective (albeit small) studies of patients with APS and prior venous thrombosis suggest that this patient's annual risk for recurrence may be as high as 50% to 67% per year after anticoagulants are discontinued.4,6 Natural history studies suggest that if a patient experiences recurrent thrombosis, the second event is more likely to occur in the same vascular system (ie, arterial or venous) as the first.15,16 Unless a patient such as this one were a poor candidate for anticoagulation (eg, not compliant with routine monitoring or has a particularly high risk of major or life-threatening bleeding), we would recommend indefinite therapeutic-dose vitamin K antagonist therapy. It should be noted that lifelong anticoagulation, especially in a young person, is associated with significant inconvenience, cost, and a risk of bleeding that accumulates with time. However, we know of no high-quality published evidence that clearly defines a time interval beyond which the risks of anticoagulation outweigh the benefits.
Warfarin management in patients with aPL can be complicated by artifactual elevation of the INR. This effect is dependent upon the type of instrument and thromboplastin that are used to measure the INR and can be avoided in most patients by simply selecting an INR reagent that is insensitive to the effect of the aPL.17-20 If such a reagent cannot be identified, an alternate method to judge the degree of oral anticoagulant-associated suppression of the coagulation cascade (such as functional factor II or X levels) will be required. In general, if such assays are used, we suggest that a factor II activity equal to 20% to 25% of normal may be an appropriate target range.
Whether follow-up (eg, annual) aPL testing should be performed after a patient such as this one has met laboratory criteria on 2 occasions 12 weeks apart is not known. We do not typically perform further testing on patients who meet criteria for APS, however we acknowledge it is possible that such testing, if it were to be repeatedly “negative,” might indicate the patient is no longer at increased risk for VTE. Further evidence describing the clinical importance of a “resolved” aPL is needed; at present, and in the absence of such evidence, we would recommend indefinite duration therapy in patients with APS irrespective of the patient's current antibody profile.2
Four months after her initial APS laboratory testing, repeat APS testing yields a second positive assay for LAC. Six months later, this patient returns to the clinic after a positive pregnancy test performed within the last 24 hours. She estimates herself to be at 6 weeks gestation.
Question: What is the optimal type, intensity, and duration of anticoagulant therapy?
In addition to arterial and venous thrombosis, women with the APS are at risk for fetal loss and premature birth. For women with aPL and a history of recurrent pregnancy loss (but no prior thrombosis), consensus guidelines propose minidose or moderate dose unfractionated heparin (UFH; eg, 10 000 to 15 000 units per day in divided doses) or prophylactic doses of LMWH (to achieve an anti–factor Xa level of 0.1 to 0.3 U/mL) in combination with aspirin21 be considered. Such therapy is, however, not without risks and its benefit remains controversial.22 Management of the nonthrombotic manifestations of aPL has been recently reviewed and will not be further discussed here.23-25
This patient, however, is at significant risk for recurrent large-vessel venous thrombosis during and after her pregnancy if anticoagulants are not administered. Because warfarin and other vitamin K antagonists are relatively contraindicated in pregnancy (especially during organogenesis, weeks 6-12), this patient should be treated with “therapeutic doses” of either subcutaneous LMWH (eg, 100 anti-Xa unit/kg every 12 hours) or unfractionated heparin (UFH). For patients with a normal baseline aPTT, UFH should be titrated to achieve an aPTT = 1.5 to 2.5 times control. For a patient whose aPTT is elevated before anticoagulation is administered, we recommend (without strong evidence) using unfractionated heparin levels targeted to an anti–factor Xa activity of around 0.5 U/mL as a gauge for dose adjustment. In either case, plasma should be sampled approximately 4 hours following a subcutaneous injection of UFH. UFH or LMWH will not only offer thromboprophylaxis, but, if combined with low-dose aspirin (75 mg to 162 mg/day), may also reduce the likelihood of pregnancy-associated complications.21,22,26,27 The impact of any anticoagulant on the risk of peripartum hemorrhage and/or use of epidural anesthesia must be weighed against potential benefits, especially when the mother is near delivery. Case reports of serious CNS injury have led at least one manufacturer of LMWH to include a “boxed warning” about the use of regional anesthesia in patients receiving its drug.28 A consensus statement published by the American Society of Regional Anesthesia suggests that such therapy is safe, if used with care in properly selected patients.29 Risk factors associated with spinal hematoma during LMWH use include the following: advanced age, concomitant antiplatelet therapy, and traumatic needle/catheter placement. Perhaps the most important factor to influence bleeding risk is the interval between LMWH injection and the time of needle/catheter placement or removal; consultation with an experienced anesthesiologist is advisable. Her vitamin K antagonist should be resumed after delivery; warfarin sodium is safe in the breastfeeding mother.30 During reinstitution of warfarin treatment, therapeutic-dose heparin or LMWH should be provided until the INR is therapeutic.21
Case 2
A 42-year-old male executive presents with dense left hemiplegia. Symptoms began about 8 hours before presentation to the casualty department. Magnetic resonance imaging (MRI) demonstrates early changes consistent with an infarct in the right internal capsule. The patient smokes heavily, is hyperlipidemic, and has a very strong family history of premature coronary artery disease. Carotid artery ultrasound and transesophageal echocardiography do not demonstrate abnormalities that explain this patient's stroke. Initial laboratory examination reveals an aCL level of 32 GPL units (normal less than 25 units). The LAC is negative. On repeat testing 12 weeks after presentation, the aCL level has returned to normal.
Question: What is the optimal type, intensity, and duration of long-term anticoagulant therapy?
This patient should be treated with antiplatelet therapy such as aspirin at a dose of at least 81 mg once daily. Aggressive efforts at smoking cessation and lipid lowering should also be undertaken.
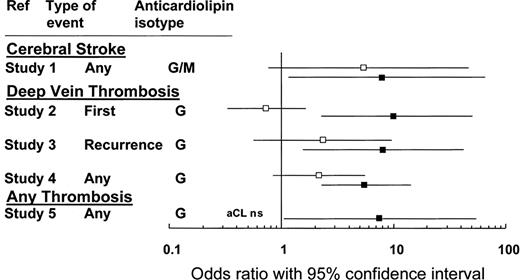
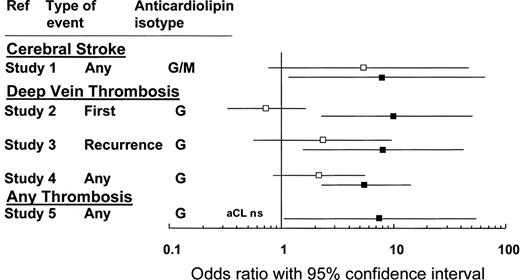
Although some authors have recommended that patients with “well-documented prothrombotic disorders” receive anticoagulation over antiplatelet therapy following a noncardioembolic stroke,31 the risks and benefits of anticoagulation with a vitamin K antagonist in this patient should be considered carefully. First, the patient's aCL level, though elevated, was not greater than the value established by internationally agreed upon criteria (Table 1). Although these criteria are based largely upon expert opinion, we suggest (in the absence of further evidence) that the link between thrombosis risk and a transient, modestly elevated aCL level (particularly in a patient with a negative lupus anticoagulant test) is not sufficiently established to justify the risk of bleeding associated with anticoagulant therapy (Figure 1).
This figure compares the association with thrombosis for aCL (□) and the LAC (■). The odds ratios (and 95% CIs) are calculated from 5 different studies involving 753 patients and 234 controls.11 NS indicates not significant. Adapted from Galli et al11 and reprinted with permission.
Current guidelines recommend that antiplatelet therapy (eg, aspirin, aspirin + dipyridamole, clopidogrel) be used instead of anticoagulation for most patients with noncardioembolic stroke.31 This recommendation stems, in part, from the results of the Warfarin-Aspirin Recurrent Stroke Study (WARSS), in which 2206 patients with noncardioembolic ischemic stroke were randomly assigned to receive either aspirin (325 mg) or warfarin (target INR = 1.4-2.8) daily in a double-blinded fashion. The primary end point, death or recurrent ischemic stroke, was reached by 196 (17.8%) of 1103 patients assigned to warfarin and 176 (16.0%) of 1103 assigned to aspirin (P = .25; hazard ratio comparing warfarin with aspirin, 1.13; 95% confidence interval [CI], 0.92 to 1.38).32
Even if the treating physician concluded that this patient has APS (based on clinical and laboratory presentation), the Antiphospholipid Antibodies and Stroke Study (APASS),33 a prospective cohort study within the larger WARSS study,32 may be relevant to this case. When the relative risk (RR) for experiencing death or recurrent stroke was calculated among 1770 patients who had experienced a stroke and who had a single positive aPL test, there was no difference between patients who were aPL positive and those who were aPL negative whether treated with warfarin (RR, 0.99; 95% CI, 0.75-1.31) or aspirin (RR, 0.94; 95% CI, 0.70-1.28).
Case 3
A 32-year-old woman who has pulmonary sarcoidosis is referred prior to elective total hip arthroplasty required as a result of steroid-associated avascular necrosis. During the evaluation for autoimmune causes of her pulmonary disease the patient had aPL screening and was found to have a positive LAC test and an aCL level that was elevated at 62 GPL units. The patient has never been pregnant and has no known prior history of thrombosis.
Question: What is the optimal type, intensity, and duration of antithrombotic prophylaxis?
Although patients with aPL who have not had prior thromboembolism are at increased risk of thrombosis, the absolute risk of clinical thromboembolism appears to be low.34 Thus, it is our opinion that the risks, inconvenience, and expense of routine therapeutic anticoagulation (ie, primary prophylaxis) in such individuals cannot be justified. At least one consensus conference has suggested that aspirin (81 mg/day) be considered in cases where aPL is identified incidentally,35 but this practice is based on retrospective findings36,37 that need confirmation in prospective, controlled trials.
For the patient presented here, the positive test for aPL does not alter the recommendation that she receive pharmacologic thromboprophylaxis following total hip arthroplasty. Even patients with no known history of aPL face a significant risk of venous thromboembolism (VTE) when undergoing hip replacement surgery.38 Thus, this patient should receive one of several pharmacologic regimens that have been shown to substantially reduce the risk of VTE after hip arthroplasty: LMWH (at a “prophylaxis” dose), fondaparinux (2.5 mg daily) started 6 to 8 hours after surgery, or warfarin (target INR = 2-3; started either the evening before or the evening after surgery). Based on evidence that extended use of thromboprophylaxis reduces the risk of VTE without substantial risk, we would recommend that this patient receive 28 to 35 days of LMWH, warfarin, or fondaparinux.39-41
Case 4
A 24-year-old woman presents to the casualty department with multiple cerebral infarcts. Carotid artery ultrasound is normal but a transthoracic echocardiogram shows “vegetations” on the mitral valve. The patient does not have fever and multiple blood cultures fail to grow organisms. At the time of admission, she is receiving atenolol and warfarin. The warfarin therapy (assigned target INR = 2.0-3.0) was initiated about 2 years previously at the time of an objectively confirmed pulmonary embolism. Her INR is 2.8 on admission. Her LAC testing has been positive on repeated evaluations.
Question: What is the optimal initial and long-term anticoagulant management of her cerebral infarction(s)?
This patient has experienced a well-described complication of the APS, nonbacterial thrombotic endocarditis (with an embolic “shower” to the brain). There is very little evidence available to guide the clinician faced with an APS patient who has suffered thrombosis despite therapeutic anticoagulation. Options commonly considered include increasing the target INR (eg, to 3.0-4.0) with or without adding an antiplatelet agent or adding aspirin without changing the target INR. Substituting therapeutic doses of an alternate anticoagulant (for example, LMWH for warfarin) either temporarily or indefinitely has also been proposed42 ; however, this option is costly (especially in North America). Although there is evidence that long-term LMWH use may increase the risk of osteoporosis,43,44 several studies have failed to demonstrate an association between LMWH use and clinically significant declines in bone mineral density.45-47 The association (or lack thereof) between LMWH and osteoporosis is particularly important for the many APS patients who have received prior corticosteroids in the treatment of their underlying autoimmune conditions.
In a recently published case series, 46 patients underwent hematopoietic stem cell transplantation (HSCT) for “refractory” SLE. Twenty-eight of these individuals also had a diagnosis of APS prior to their transplantation. The authors reported that laboratory evidence of aCL and LAC disappeared in many of these patients and that 18 of 22 APS patients refractory to chronic anticoagulation successfully discontinued anticoagulation therapy a median of 4 months after transplantation.48 Another group of investigators has reported favorable outcomes after using rituximab in 3 APS patients considered resistant to conventional medications.49 Both HSCT and rituximab would need to be further studied before widespread use could be recommended for any form of APS.
In a case such as this one, our recommendations would be either to increase the target INR value (to 3.0-4.0) and add aspirin 81 mg daily, or to treat the patient with extended duration weight-adjusted, therapeutic-dose low-molecular-weight heparin. Both increased target intensity of anticoagulation as well as prescribing concomitant antiplatelet therapy increase the relative risk of bleeding compared with vitamin K antagonist monotherapy with a target INR of 2.5.50-52 Simultaneously adding aspirin and increasing the target INR likely results in a greater risk of bleeding than either strategy would alone. Although published experience is limited to 3 to 6 months, the risk of hemorrhage associated with long-term, therapeutic doses of LMWH appears to be comparable with the corresponding risk among patients treated with warfarin monotherapy (target INR of 2.5).42,53,54 The maximum duration of therapeutic-dose low-molecular-weight heparin therapy has not been defined; published experience suggests patients may be treated as long as 6 years without overt complications.42
Case 5
You are asked to perform consultation for a 29-year-old female who presented with severe headache and has been diagnosed with objectively confirmed cavernous sinus thrombosis. Physical examination is significant for the absence of neurologic deficits. Livedo reticularis is present on the lower extremities. Initial laboratory testing reveals a slightly low platelet count and testing for LAC is positive. The patient is anticoagulated for 6 days with both LMWH and warfarin, then with warfarin alone (target INR = 2.0-3.0). Her headache improves, she is discharged with warfarin, and she returns to see you in 3 months. Repeat laboratory testing for LAC is negative, and aCL antibody levels (IgG and IgM isotypes) are lower than the upper limit of normal.
Questions: Does this patient have APS? What further testing could be used to establish the diagnosis?
There are numerous factors that make this case suspicious for APS. Among these are the patient's young age, the unusual location of her spontaneous thrombosis, and the presence of LAC on initial laboratory testing. In addition, livedo reticularis and thrombocytopenia are seen with some frequency in APS patients. Despite the numerous clues pointing toward APS in this woman, her case does not fulfill the classification criteria because repeat laboratory testing failed to demonstrate aPL.
In cases such as this, where the pretest probability of disease is high, yet the patient fails to meet “consensus criteria” for APS, the clinician may use one of several strategies. First, in light of the new classification criteria for APS8 shown in Table 1, testing for anti–β2 GP1 antibodies should be pursued because persistent positivity for such antibodies would establish the diagnosis of APS. Second, antibodies against prothrombin (aPT) can also be seen in patients with APS—at least one study has demonstrated that among patients with SLE and thrombosis, these aPTs may be present even when testing for LAC and aCL is negative.55 The clinical role of aPT antibody testing is, as yet, unclear. Repeat testing for LAC, aCL, anti–β2 GP1, or aPT might be reasonable because these levels commonly fluctuate, and a positive result might satisfy a desire (from the clinician and/or the patient) to identify a likely explanation for the events observed clinically. That being said, we would be reluctant to discontinue anticoagulation in a patient such as this one, regardless of the results. This hesitation is based primarily on our concern over the potentially devastating clinical consequences of recurrent thrombosis in this vascular distribution.
Summary
Antiphospholipid antibody syndrome is a common and potentially devastating autoimmune disorder. Evidence-based treatment recommendations for selected patients with aPL-associated thromboembolism can now be made based on published literature. However, numerous questions remain unanswered. These questions include the optimal management of patients with arterial thrombosis in the setting of persistently positive aPL, optimal management of patients who suffer recurrent thrombosis despite therapeutic anticoagulation, and refinement of current diagnostic tests to allow more confident assignment of patients with suspected APS.
Authorship
Contribution: D.A.G., M.A.K., and M.A.C. conceived and wrote the paper. All have seen and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark A. Crowther, Room L208, St Joseph's Hospital, 50 Charlton Ave East, Hamilton, ON L8N 4A6; e-mail: crowthrm@mcmaster.ca.