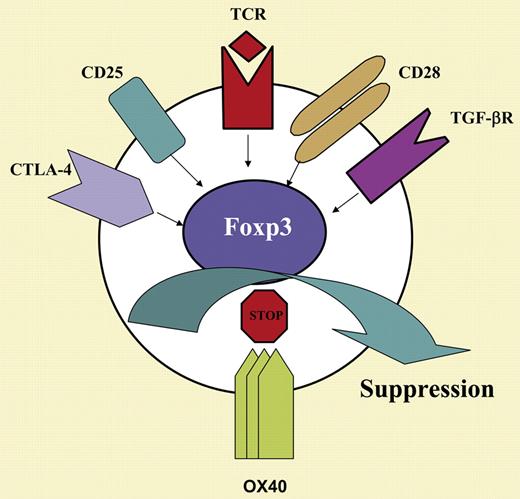
Immune regulation is central to T-cell responses and the acquisition of tolerance. A subset of CD4+CD25+ T cells that are highly specialized in regulatory functions expresses a transcription factor, Foxp3; the cells comprising this subset are called Foxp3+ Tregs. Such Foxp3+ Tregs are dedicated to the creation and maintenance of immune tolerance by controlling cytopathic effector T cells.1 Phenotypically, activated effector T cells and Foxp3+ Tregs express a plethora of cell-surface receptors, some of which are identical on both subsets (see the figure). Thus, a better appreciation of how the same receptors regulate functionally divergent T-cell subsets is an important and clinically relevant issue.
OX40 (CD134) is one such cell-surface receptor that can be expressed by both effector T cells and Tregs.2 Although OX40, a member of the TNF/TNF-R superfamily, has been extensively studied as a potent costimulatory molecule to activated effector T cells,3 its putative role in regulating Treg function remains to be elucidated. In the current issue of Blood, Vu and colleagues detail a surprising finding: that OX40 plays a dual role in the T-cell response, and may inhibit and negatively regulate Foxp3+ Tregs. In a series of well-designed and elegant experiments using a newly developed Foxp3-GFP reporter model, the authors discovered that Foxp3+ Tregs are present in normal numbers in OX40-deficient mice and, when selected and tested in vitro, they do function just as well as WT Foxp3+ Tregs in suppressing effector T-cell activation. Thus, both development and function of Foxp3+ Tregs appear to be OX40 independent. Interestingly, stimulation of OX40 on mature Foxp3+ Tregs completely abolished the ability of Tregs to suppress effector T-cell proliferation and cytokine production. Moreover, OX40 signaling to Tregs does not seem to affect their survival and proliferation, but appears to repress the expression of Foxp3, which is a striking feature of this model. Importantly, the induction of Foxp3 expression in freshly activated effector T cells by TGF-β was consistently prevented by OX40. Thus, OX40 represents a potent negative regulator not only to natural Foxp3+ Tregs but also to Tregs generated de novo from activated effector T cells, suggesting that OX40 controls an important checkpoint in Treg homeostasis.
The findings of Vu and colleagues imply that, unlike other costimulatory molecules, OX40 plays a dual role in T-cell activation. It is undoubtedly a potent costimulatory molecule to effector T cells, but it is also a powerful negative regulator of Foxp3+ Tregs. Indeed, OX40 signaling has been recently shown to shut off IL-10–producing CD4+ type 1 regulatory T (Tr1) cells.4 However, many questions remain to be addressed. Clearly, OX40 can disarm Foxp3+ Tregs, but it remains unclear whether this effect is reversible or permanent. In addition, the phenotype and function of effector T cells that are supposed to become new Foxp3+ Tregs but are prevented by OX40 require careful investigation. Given the close relationship between inducible Tregs and Th17 cells,5 the possibility of Th17 induction by OX40 needs to be examined. Finally, the molecular fingerprints as to how OX40 signals functionally different effector T cells and Foxp3+ Tregs need to be established. Nonetheless, the findings of Vu and colleagues are significant and may have far-reaching clinical ramifications, as manipulation of Foxp3+ Tregs is clearly an attractive approach in transplantation tolerance, autoimmunity, and cancer treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■