Abstract
Aging in mice and humans is characterized by declining T-lymphocyte production in the thymus, yet it is unclear whether aging impacts the T-lineage potential of hematopoietic progenitors. Although alterations in the lymphoid progenitor content of aged mouse bone marrow (BM) have been described, irradiation-reconstitution experiments have failed to reveal defects in T-lineage potential of BM hematopoietic progenitors or purified hematopoietic stem cells (HSCs) from aged mice. Here, we assessed T-progenitor potential in unmanipulated recipient mice without conditioning irradiation. T-progenitor potential was reduced in aged BM compared with young BM, and this reduction was apparent at the earliest stages of intrathymic differentiation. Further, enriched populations of aged HSCs or multipotent progenitors (MPPs) gave rise to fewer T-lineage cells than their young counterparts. Whereas the T-precursor frequency within the MPP pool was unchanged, there was a 4-fold decline in T-precursor frequency within the HSC pool. In addition, among the T-competent HSC clones, there were fewer highly proliferative clones in the aged HSC pool than in the young HSC pool. These results identify T-compromised aged HSCs and define the nature and cellular sites of prethymic, age-related defects in T-lineage differentiation potential.
Introduction
Aging in mice and humans is characterized by declining T-lymphocyte production by the thymus.1 This is associated with impaired immune function in the elderly.2 Further, age-related decline in thymic function is a barrier to effective recovery of the peripheral T-cell compartment after lymphocyte-depleting events, such as chemotherapy or HIV infection.3,4 Despite its clinical importance, cellular mechanisms governing age-related loss of thymic function are not well defined. Alterations in thymic stromal cells do occur,5,6 but whether aging impacts bone marrow (BM)-derived hematopoietic progenitors is unclear.
Previously, the earliest progenitor within the mouse thymus was thought to be in the double-negative 1 (DN1; CD4− CD8− CD44+ CD25−) population.7 Because this population is intact in aged thymi, an intrathymic block in the development of T-cell progenitors after the DN1 stage was predicted.8 However, it is now established that the earliest progenitors in the thymus are a population of CD4lo lineage-marker− CD25− c-Kithi cells termed early T-lineage progenitors (ETPs) that only partially overlap with the DN1 population.9 ETPs in aged thymi are reduced compared with young thymi,10,11 thus raising the possibility that prethymic progenitors upstream of ETPs in the BM are defective in producing T cells.
Several studies have shown alterations in lymphoid progenitor content in aged BM, including a lower frequency of multipotent progenitors (MPPs).12,13 Subsets of MPPs are thought to settle within the thymus,14 so reduced MPP frequency in aged BM predicts that T-progenitor potential will also be reduced. However, whether there are functional defects in aged BM progenitors remains unclear.15 Aged BM administered intravenously reconstitutes the thymus of irradiated mice with slower kinetics than young BM but eventually produces similar numbers of thymocytes.16,17 Studies using fetal thymic organ culture showed that aged BM is less efficient at reconstituting thymic lobes compared with young BM in competitive conditions.18 It is possible that these data reflect reduced MPP frequency in aged BM, since MPPs can rapidly develop into thymocytes. However, because these studies did not assess purified populations, they did not distinguish between defects within individual hematopoietic progenitor cells versus age-related changes in frequencies of hematopoietic progenitor populations in the BM preparations.
Reduced MPP frequency in aged BM suggests that there may be upstream functional defects in hematopoietic stem cells (HSCs). Multiple studies using long-term irradiation-reconstitution models to assess aged HSCs in competitive conditions suggested that lymphoid differentiation may be impaired, but these results are difficult to collectively interpret. Reconstitution of lethally irradiated mice with mixtures of old and young BM cells did not reveal any competitive defects in the ability of aged cells to reconstitute peripheral T and B cells, suggesting intact T potential of aged HSCs.19–21 Experiments using small numbers of purified aged HSCs to competitively reconstitute irradiated mice have shown defects in B-lineage but not T-lineage reconstitution.13 A similar study reported reduced B- and T-lymphoid reconstitution relative to myeloid reconstitution by aged HSCs, but this was never compared with young HSCs.20 Thus, whether aged HSCs are intrinsically defective at giving rise to T lymphocytes in vivo remains an open question.
In this study, we establish that aged BM progenitors are less efficient at giving rise to T-lineage cells by using experimental conditions that do not involve irradiation. We show that aged unfractionated BM, purified MPPs, and purified HSCs produce fewer T-lineage cells than their young counterparts when assessed in vitro and in vivo in young host animals. Limit-dilution analysis of purified HSCs in vitro revealed that a lower fraction of aged HSCs possessed T potential. Additionally, single-cell analysis showed that aged T-competent HSCs gave rise to fewer T-lineage cells. In contrast, aged MPPs did not have a lower T-precursor frequency even though they produced fewer T-lineage cells in bulk assays. These data suggest that T-compromised HSCs do not transition to the downstream MPP pool and that the smaller burst size of T-competent HSCs is also reflected in their MPP progeny. This work therefore demonstrates that multiple age-related defects in T potential occur within the HSC population.
Materials and methods
Mice
Two-month-old and 18- to 24-month-old C57BL/6 female mice were obtained from the National Institute on Aging (NIA). No differences in progenitors were observed between 18 months and 24 months of age, so data from these groups were combined. C57BL/6 Ly5SJL mice from the National Cancer Institute were used as host mice in all irradiation transplantation experiments. To overcome minor histoincompatibilities between NIA mice and congenic C57BL/6 Ly5SJL mice during nonirradiated transplantation experiments, the 2 lines were crossed to yield F1 C57BL/6 Ly5B6xLy5SJL progeny, which served as host mice. For irradiation-reconstitution experiments, recipient mice were lethally irradiated (9 Gy) and intravenously injected with 1:1 mixtures of whole BM (3 × 105 cells each). Experiments were performed with Institutional Animal Care and Use Committee (IACUC) approval.
Cell preparation
Thymi were harvested and dissociated into single-cell suspensions, and total cellularity was calculated after obtaining cell counts with a hemacytometer. An aliquot was set aside, and the remaining cells were depleted of CD4hi and CD8hi cells using anti-CD4 (GK1.5) and anti-CD8 (53-6.7) and anti-rat IgG-conjugated magnetic beads (Qiagen, Hilden, Germany). BM was flushed from the hind limbs and red blood cells (RBCs) were lysed using ACK lysing buffer (Cambrex, Walkersville, MD). For nonirradiated transplantation experiments, BM was depleted of T lymphocytes using anti-CD4 and anti-CD8 antibodies to prevent graft-versus-host disease and also depleted of B220+ B-lymphocyte progenitors for accurate assessment of donor pro-B-cell (B220+ CD43+ CD19+ AA4.1+) production after transplantation. Peripheral blood was collected retro-orbitally.
Flow cytometry
All antibodies were from BD Pharmingen (San Jose, CA) or Ebiosciences (San Diego, CA). For phenotypic analysis of cells, the following antibodies were used: FITC-conjugated CD45.1 (A20); PE-conjugated CD43 (S7), Gr-1 (8C5), CD4 (GK1.5), Thy-1.2 (53-2.1); biotinylated CD19 (1D3), CD25 (PC61), Flt3 (A2F10); APC-conjugated B220 (RA3-6B2), CD11b (M1/70), CD8 (53-6.7), c-Kit (2B8); PECy5.5-conjugated CD45.2 (104), B220 (RA3-6B2), Sca-1; PECy7-conjugated AA4.1, c-Kit (2B8); and APC-Alexa 750-conjugated CD25 (PC61). Biotinylated antibody was visualized with streptavidin-APC. To exclude mature cells, a lineage cocktail of the following FITC- or PE-conjugated antibodies was used: B220 (RA3-6B2), CD19 (1D3), CD11b (M1/70), Gr1 (8C5), CD11c (HL3), NK1.1 (PK136), Ter119, CD8α (53-6.7), CD8β (53-5.8), TCRγδ (GL-3), TCRβ (H57), and CD3ϵ (2C11). Dead cells were excluded with 4,6-diamidino-2-phenylindole (DAPI). Cells were analyzed on a 4-laser LSR II (Becton Dickinson, San Jose, CA) and data were analyzed using FlowJo version 4.6.2 (Tree Star, Ashland, OR). Cells were sorted on a FACSAria (Becton Dickinson) and sort purity was routinely checked.
Real-time quantitative polymerase chain reaction (PCR)
mRNA from sorted populations was isolated using the RNeasy kit (Qiagen) and reverse transcribed with Superscript II (Invitrogen, Carlsbad, CA). Resultant cDNAs were amplified and detected using premade FAM-labeled probes purchased from Applied Biosystems (Foster City, CA). Amplification and analysis were carried out on a 7900HT Fast Real-Time PCR System (Applied Biosystems).
OP9-DL1 culture
OP9-DL1 cells were kindly provided by J. C. Zúñiga-Pflücker, and cultures were carried out as described.22 For bulk assays, 200 to 250 purified HSCs or MPPs were plated per well of a 24-well plate. For limit-dilution and single-cell experiments, 20, 10, 5, or 1 HSCs or MPPs were plated per well in 96-well plates. In some burst-size experiments, 5 HSCs were plated per well to increase colony formation. The probability of monoclonality of T colonies from such wells is at least 0.84.23 For all cultures, Flt3 ligand was added at a final concentration of 5 ng/mL and IL-7 at 1 ng/mL. Hematopoietic cells were transferred to fresh stromal layers with fresh cytokines at days 8 to 10 of culture. HSC cultures were harvested for analysis at days 18 to 20 and MPPs at days 8 to 10. Numbers of cells produced per well were obtained by resuspending each well in a constant volume and counting on a flow cytometer at a constant flow rate for a defined period of time. Absolute cell numbers were calculated from the cytometry data using a standard curve generated with known numbers of cells.
Intrathymic injections
Two thousand purified progenitors or 2.5 × 105 to 5 × 105 whole BM cells were injected into the thymi of nonirradiated anesthetized F1 mice.
Statistics
Poisson statistics were calculated using L-Calc version 1.1 software (Stem Cell Technologies, Vancouver, BC, Canada).
Results
Reduced T potential of aged progenitors
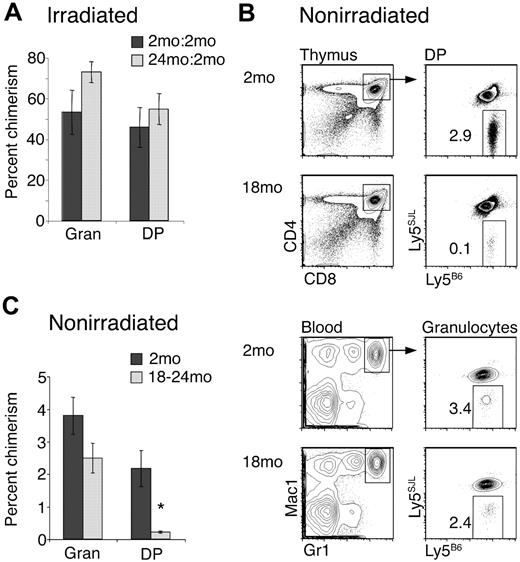
Previous studies have not detected defects in T-lineage potential of aged progenitors using BM chimeras, but these earlier studies examined peripheral T-cell reconstitution rather than thymus reconstitution.19,20 Thus it was possible that reduced T-lineage differentiation by aged progenitors was masked by homeostatic proliferation of aged progenitor-derived T cells in the periphery. To address this, we reconstituted lethally irradiated mice with mixtures of equal numbers of 2-month-old Ly5SJL and 18- to 24-month-old Ly5B6 whole BM. As controls, we mixed 2-month-old Ly5B6 with 2-month-old Ly5SJL BM. Mice were analyzed at least 10 weeks later for granulocyte and CD4+ CD8+ double-positive (DP) thymocyte chimerism. Thymocyte production by aged progenitors in radiation chimeras was similar to that by young progenitors (Figure 1A), consistent with previous work.19,20
Aged BM is inefficient at producing T-lineage cells in vivo in nonirradiated recipients. (A) Lethally irradiated (9 Gy) Ly5SJL recipients were intravenously injected with 1:1 mixtures (3 × 105 cells each) of 18- to 24-month-old Ly5B6 BM and 2-month-old competitor Ly5SJL BM (▒). Control chimeras (░) were generated by injecting mixtures of 2-month-old Ly5B6 and 2-month-old competitor Ly5SJL BM. Thymocyte and granulocyte populations were analyzed for chimerism at least 10 weeks later. Bars represent percentages of Ly5B6 cells within each population. Absolute numbers of thymocytes produced by Ly5B6 BM were also similar (young: 77.1 × 106 ± 19.3 × 106; aged: 64.7 × 106 ± 12.8 × 106; P = .3). Data represent 11 chimeras in which 2-month-old BM was mixed with 2-month-old BM, and 22 chimeras in which 2-month-old BM was mixed with 18- to 24-month-old BM. Differences between young and old donors in the DP compartment were not significant. Error bars represent 1 SEM. (B) T-depleted BM cells (20 × 106) from 2-month-old or 18- to 24-month-old Ly5B6 donors were injected into nonirradiated 5-week-old Ly5B6xLy5SJL recipient mice. Mice were analyzed 13 to 16 weeks later for donor-derived chimerism in the DP thymocyte and blood granulocyte compartments. (C) Summary of data shown in panel B, representing 4 to 6 mice per group. Error bars represent 1 SEM (*P < .01). Absolute numbers of donor-derived thymocytes were also significantly different (young: 1.58 × 106 ± 0.17 × 106; aged: 0.32 × 106 ± 0.054 × 106; P < .001).
Aged BM is inefficient at producing T-lineage cells in vivo in nonirradiated recipients. (A) Lethally irradiated (9 Gy) Ly5SJL recipients were intravenously injected with 1:1 mixtures (3 × 105 cells each) of 18- to 24-month-old Ly5B6 BM and 2-month-old competitor Ly5SJL BM (▒). Control chimeras (░) were generated by injecting mixtures of 2-month-old Ly5B6 and 2-month-old competitor Ly5SJL BM. Thymocyte and granulocyte populations were analyzed for chimerism at least 10 weeks later. Bars represent percentages of Ly5B6 cells within each population. Absolute numbers of thymocytes produced by Ly5B6 BM were also similar (young: 77.1 × 106 ± 19.3 × 106; aged: 64.7 × 106 ± 12.8 × 106; P = .3). Data represent 11 chimeras in which 2-month-old BM was mixed with 2-month-old BM, and 22 chimeras in which 2-month-old BM was mixed with 18- to 24-month-old BM. Differences between young and old donors in the DP compartment were not significant. Error bars represent 1 SEM. (B) T-depleted BM cells (20 × 106) from 2-month-old or 18- to 24-month-old Ly5B6 donors were injected into nonirradiated 5-week-old Ly5B6xLy5SJL recipient mice. Mice were analyzed 13 to 16 weeks later for donor-derived chimerism in the DP thymocyte and blood granulocyte compartments. (C) Summary of data shown in panel B, representing 4 to 6 mice per group. Error bars represent 1 SEM (*P < .01). Absolute numbers of donor-derived thymocytes were also significantly different (young: 1.58 × 106 ± 0.17 × 106; aged: 0.32 × 106 ± 0.054 × 106; P < .001).
Irradiation is associated with increased production of cytokines and chemokines, and transplanted BM progenitors undergo rapid proliferation in irradiated recipients.24,25 Thus, it was possible that changes in aged progenitor function were masked after exposure to the irradiated environment. To address this possibility, nonirradiated Ly5B6xLy5SJL F1 recipient mice received 20 × 106 T-depleted BM cells from 2-month-old or 18- to 24-month-old mice and were analyzed 13 to 16 weeks later for production of granulocytes and committed T precursors in the thymus. At this late time point, production of granulocytes and committed T precursors reflects the activity of HSCs in the donor inoculum.11,14,26 Analysis of blood granulocytes showed that aged and young BM engrafted at a comparable level, with approximately 3% chimerism, as expected in nonirradiated recipients.14,27 Interestingly, aged BM gave rise to approximately 10-fold fewer DP thymocytes than young BM (Figure 1B,C). There was a slight drop in myeloid chimerism, but this was not statistically significant. Hence, defects in T-lineage potential are present in aged HSCs and can be revealed in nonirradiated hosts.
Altered progenitor content of aged BM
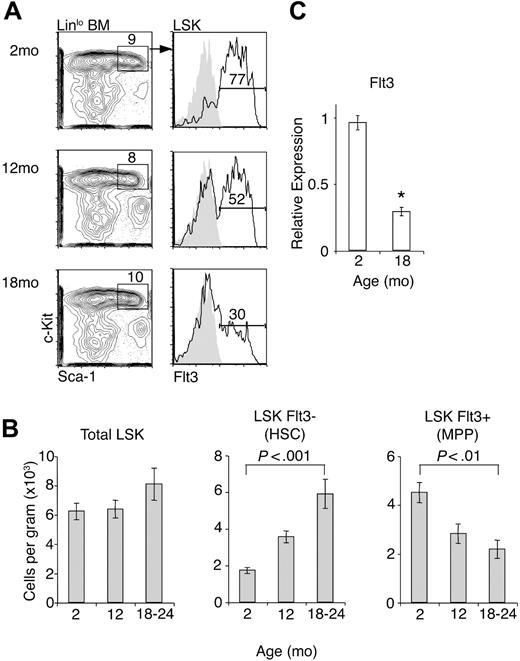
Progenitors introduced intravenously must undergo 2 distinct processes to give rise to thymocytes: (1) migration into the thymus, and (2) response to intrathymic inductive signals to differentiate along the T pathway.11 We have recently shown that HSCs do not directly settle in the thymus of nonirradiated recipients, but instead the thymus is likely settled, at least in part, by Ccr9-expressing cells contained within the MPP population.14 Therefore, the mechanism responsible for the apparent loss of T potential in vivo could be either a decreased generation of thymus-settling progenitors or decreased intrathymic expansion of progenitors. To determine if thymus-settling progenitors were reduced in aged BM, we examined the frequencies of Linlo Sca-1+ c-Kithi (LSK) cells. These LSK cells consist of a Flt3− subset highly enriched in HSC and a Flt3+ subset, which is nonrenewing MPP.26 There was a reduced frequency of Flt3+ cells within the LSK population at 12 months of age and this decreased further by 18 months of age (Figure 2A). This correlated with reduced expression of Flt3 transcripts in LSK cells of aged mice (Figure 2C). Absolute numbers of total LSK cells were stable but numbers of HSCs were increased and numbers of MPPs were decreased with age (Figure 2B), consistent with previous work.13,19,28 We also examined Ccr9 expression on the MPP population by flow cytometry and quantitative PCR to see if thymus-settling cells were further reduced within this subset. We did not find significant differences in the expression of Ccr9 in young and aged MPPs (data not shown). These results show that frequencies and numbers of HSCs and MPPs are consistently altered in aging BM. As expected from previous work, we also found a reduction in downstream early B-lineage progenitors (Lin− c-Kitlo IL-7Rα+ AA4.1+ Flt3+; data not shown).12
Altered frequencies of HSCs and MPPs in aged BM. (A) BM was harvested from 2-month-old, 12-month-old, or 18- to 24-month-old mice and LSK cells were analyzed for Flt3 expression by flow cytometry. (B) Numbers of HSCs (defined as LSKFlt3−) and MPPs (defined as LSKFlt3+) in young and aged BM, calculated from flow cytometric plots in panel A. Numbers are expressed as total cells (counted from 2 femora plus 2 tibiae plus 2 humeri from each mouse) per gram of body weight. Data represent at least 6 mice per group. Error bars represent 1 SEM. (C) cDNA from total LSK cells was amplified in the presence of gene-specific FAM-labeled Taqman probes. Data are shown as expression relative to Hprt amplification (*P < .001). Error bars represent 1 SEM.
Altered frequencies of HSCs and MPPs in aged BM. (A) BM was harvested from 2-month-old, 12-month-old, or 18- to 24-month-old mice and LSK cells were analyzed for Flt3 expression by flow cytometry. (B) Numbers of HSCs (defined as LSKFlt3−) and MPPs (defined as LSKFlt3+) in young and aged BM, calculated from flow cytometric plots in panel A. Numbers are expressed as total cells (counted from 2 femora plus 2 tibiae plus 2 humeri from each mouse) per gram of body weight. Data represent at least 6 mice per group. Error bars represent 1 SEM. (C) cDNA from total LSK cells was amplified in the presence of gene-specific FAM-labeled Taqman probes. Data are shown as expression relative to Hprt amplification (*P < .001). Error bars represent 1 SEM.
Reduced T potential of aged thymus-settling progenitors
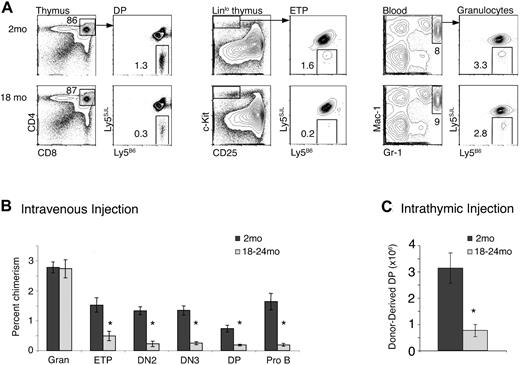
The above data indicate that the frequency of MPPs is reduced in aged BM. As these subsets contain progenitors able to directly migrate into the thymus and initiate T lymphopoiesis,14 these data predict that unfractionated aged BM progenitors delivered intravenously would be defective in short-term T-lymphopoietic assays. We therefore assessed the ability of aged BM to give rise to thymocytes in short-term transplantation experiments. Twenty million CD4-, CD8-, and B220-depleted BM cells from 2-month-old or 18- to 24-month-old mice were injected intravenously. Because generation of large numbers of DP thymocytes takes approximately 3 weeks in this system,14 recipient mice were analyzed 3 weeks later for the presence of donor-derived thymocytes and myeloid cells (Figure 3A). Blood granulocyte chimerism was similar in recipients of aged or young BM, but analysis of thymi revealed that donor-derived thymocyte chimerism was approximately 4-fold lower in recipients of aged BM than young BM (Figure 3B). This trend was also apparent when absolute numbers of donor-derived DP thymocytes were compared (young: 1.1 × 106 ± 1.8 × 105; old: 0.28 × 106 ± 7.2 × 104; P < .001). Further, the reduction in thymocyte chimerism in mice that had received aged BM was apparent at the ETP stage and persisted to the DP stage (Figure 3B).
Reduced T potential of aged BM in short-term assays. (A) Ly5B6 donor BM from 2 month-old and 18- to 24-month-old mice was depleted of CD4+, CD8+, and B220+ cells, and 20 × 106 of the remaining cells were injected intravenously into nonirradiated mice. Recipients were analyzed at 3 weeks for chimerism in ETP (Linlo c-Kithi CD25−), DN2 (Linlo c-Kithi CD25+), DN3 (Linlo c-Kit− CD25+), and DP (CD4+ CD8+) populations in the thymus, granulocytes in the blood (Mac-1+ Gr-1+), and Pro B (B220+ CD43+ CD19+ AA4.1+) cells in the BM by flow cytometry. (B) Summary of experiments shown in panel A. Data represent 7 mice per group across 3 independent experiments. Error bars represent 1 SEM (*P ≤ .01). Absolute numbers of donor-derived thymocytes were also significantly different (young: 1.1 × 106 ± 0.17 × 106; aged: 0.28 × 106 ± 0.072 × 106; P < .001). (C) BM cells (5 × 105 to 1 × 106) were injected into the thymi of nonirradiated mice. Thymi were harvested and analyzed for donor-derived DP thymocytes 21 days later. Data are combined from 3 independent experiments each, with 18 to 20 total mice per group (*P ≤ .001). Error bars represent 1 SEM.
Reduced T potential of aged BM in short-term assays. (A) Ly5B6 donor BM from 2 month-old and 18- to 24-month-old mice was depleted of CD4+, CD8+, and B220+ cells, and 20 × 106 of the remaining cells were injected intravenously into nonirradiated mice. Recipients were analyzed at 3 weeks for chimerism in ETP (Linlo c-Kithi CD25−), DN2 (Linlo c-Kithi CD25+), DN3 (Linlo c-Kit− CD25+), and DP (CD4+ CD8+) populations in the thymus, granulocytes in the blood (Mac-1+ Gr-1+), and Pro B (B220+ CD43+ CD19+ AA4.1+) cells in the BM by flow cytometry. (B) Summary of experiments shown in panel A. Data represent 7 mice per group across 3 independent experiments. Error bars represent 1 SEM (*P ≤ .01). Absolute numbers of donor-derived thymocytes were also significantly different (young: 1.1 × 106 ± 0.17 × 106; aged: 0.28 × 106 ± 0.072 × 106; P < .001). (C) BM cells (5 × 105 to 1 × 106) were injected into the thymi of nonirradiated mice. Thymi were harvested and analyzed for donor-derived DP thymocytes 21 days later. Data are combined from 3 independent experiments each, with 18 to 20 total mice per group (*P ≤ .001). Error bars represent 1 SEM.
This T-lymphopoietic defect could reflect reduced migration ability and/or reduced T-differentiation/expansion ability of aged progenitors. To distinguish between these, we injected BM cells directly into the thymus of nonirradiated mice, thus bypassing the migration requirement (Figure 3C). Aged BM cells maintained a defect in DP thymocyte production, indicating that they were deficient in T-lineage differentiation. Although a migration defect could not be ruled out, it was not sufficient to explain reduced thymocyte production by aged BM. Consistently, B-lineage production, which does not require thymus migration, was also decreased (Figure 3B).
Aged MPPs produce fewer T-lineage progeny
The experiments shown in Figures 1 and 2 establish reduced T potential in aged BM progenitors. We next asked if there were functional defects on a per-progenitor basis. We injected equal numbers of purified MPPs into the thymus of nonirradiated recipients and analyzed DP thymocyte production 3 weeks later (Figure 4A). Aged MPPs gave rise to 2.4-fold fewer thymocytes than young MPPs. To confirm this result in vitro, we used OP9 stromal cells expressing the Notch ligand Delta-like 1 (OP9-DL1) to generate T-lineage cells.22,29 In this system, adult BM progenitors efficiently differentiate into DN2/DN3-stage CD25+Thy-1+ cells, although further differentiation to the DP stage is inefficient.30 We cultured purified MPPs on OP9-DL1 layers and subsequently analyzed cells for expression of Mac-1, B220, CD25, and Thy-1 (Figure 4B). Although no B220+ cells were detected, substantial numbers of Mac-1+ cells were present. Production of myeloid lineage cells from young and aged MPPs was equivalent, but production of Thy-1+ CD25+ T-lineage cells by 18- to 24-month-old MPPs was decreased 3.3-fold compared with 2-month-old MPPs. Together, these data show that reduced T-lineage output is an intrinsic feature of aged MPPs.
Aged MPPs are inefficient at producing T-lineage cells in vivo and in vitro. (A) Two thousand sorted MPPs from 2-month-old or 18- to 24-month-old donors were injected into the thymi of nonirradiated recipients, and donor-derived DP progeny were analyzed 21 days later. Error bars represent 1 SEM (*P = .05). (B) Two hundred to 250 purified MPPs were plated onto OP9-DL1 stromal layers supplemented with IL-7 and FL, and numbers of T-lineage (Thy1+ CD25+) and myeloid lineage (Mac1+) progeny were assessed 8 to 10 days later. For bar graphs, error bars represent 1 SEM (*P < .01). (C) Limit-dilution analysis was performed in vitro by culture of purified 2-month-old and 18- to 24-month-old MPPs on OP9-DL1 stromal layers. Poisson statistics determined frequencies of lineage-competent cells (T lineage young: 1 in 19 [95% confidence interval 1 in 13-26], aged: 1 in 21 [1 in 15-29], P = .6; myeloid lineage young: 1 in 13 [1 in 9-17], aged: 1 in 10 [1 in 8-14], P = .3). Twenty-eight wells were analyzed for each cell dose.
Aged MPPs are inefficient at producing T-lineage cells in vivo and in vitro. (A) Two thousand sorted MPPs from 2-month-old or 18- to 24-month-old donors were injected into the thymi of nonirradiated recipients, and donor-derived DP progeny were analyzed 21 days later. Error bars represent 1 SEM (*P = .05). (B) Two hundred to 250 purified MPPs were plated onto OP9-DL1 stromal layers supplemented with IL-7 and FL, and numbers of T-lineage (Thy1+ CD25+) and myeloid lineage (Mac1+) progeny were assessed 8 to 10 days later. For bar graphs, error bars represent 1 SEM (*P < .01). (C) Limit-dilution analysis was performed in vitro by culture of purified 2-month-old and 18- to 24-month-old MPPs on OP9-DL1 stromal layers. Poisson statistics determined frequencies of lineage-competent cells (T lineage young: 1 in 19 [95% confidence interval 1 in 13-26], aged: 1 in 21 [1 in 15-29], P = .6; myeloid lineage young: 1 in 13 [1 in 9-17], aged: 1 in 10 [1 in 8-14], P = .3). Twenty-eight wells were analyzed for each cell dose.
To ask whether the defect in T-lineage output by aged MPPs could be accounted for by a lower T-precursor frequency within this population, we performed limit-dilution analysis using the OP9-DL1 system (Figure 4C). We detected no significant difference in precursor frequencies of T or myeloid cells between aged and young MPPs (T precursors: 1 in 21 aged versus 1 in 19 young, P = .6; myeloid precursors: 1 in 10 aged versus 1 in 13 young, P = .3). These results indicate that although a similar fraction of MPPs respond to T-inductive signals, aged MPPs produce fewer T-lineage progeny on a per-progenitor basis. However, producing fewer progeny is not a general feature of aging progenitors, since myeloid production is intact.
Multiple defects in the aged HSC pool
The data presented in Figures 2 and 4 establish that there are fewer MPPs in aged BM and that they are impaired in T-lineage differentiation. We wished to determine if defective MPP generation and function emanated from defects in upstream HSCs. To directly test the T potential of aged HSCs, we cultured purified HSCs on OP9-DL1 and analyzed numbers of T and myeloid progeny (Figure 5A). Production of Thy-1+ CD25+ T-lineage cells from 18- to 24-month-old HSCs was decreased compared with production from 2-month-old HSCs. In contrast, myeloid production by aged HSCs was increased. Thus, the T-lineage differentiation defect observed in long-term nonirradiated transplant recipients (Figure 1C) is reproducible in vitro, consistent with the defect being intrinsic to HSCs. Intrathymic injection assays also showed reduced thymocyte production by aged HSCs (data not shown). Together, these results indicate that a loss of T potential occurs in the HSC compartment and that this change is cell intrinsic, as it is evident ex vivo (Figure 5A) and in vivo in young hosts (Figure 1B).
The aged HSC pool has a lower frequency of T-competent cells and fewer highly proliferative T-competent clones. (A) Two hundred fifty sorted 2-month-old or 18- to 24-month-old HSCs were plated on OP9-DL1 stromal layers and analyzed on days 17 to 19. Data shown represent 5 independent experiments (*P < .001). (B) Limit-dilution analysis was performed on purified HSCs in vitro by OP9-DL1 coculture. Poisson statistics determined frequencies of lineage-competent cells (T lineage young: 1 in 15 [95% confidence interval 1 in 11-22], aged: 1 in 57 [1 in 33-99], P < .001; myeloid lineage young: 1 in 10 [1 in 7-14], aged: 1 in 14 [1 in 10-19], P = .2). Twenty-four wells were analyzed for each cell dose. (C) Single HSCs were plated on OP9-DL1 layers with cytokines and analyzed by flow cytometry 18 days later. Data shown are restricted to those clones that generated detectable T-lineage progeny and are thus T competent. Data were analyzed using the binomial distribution probability mass function. Colonies with greater than 10 000 progeny were considered highly proliferative (denoted by dashed line), and the frequency of highly proliferative T-competent clones was greater in the young HSC pool than aged (young: 25 highly proliferative colonies out of 50 total T colonies; aged: 10 out of 32; P = .015). Differences were also significant when other arbitrary cutoff values from 5000 to 100 000 were used. For the myeloid lineage, aged HSCs had a higher frequency of highly proliferative myeloid producing clones compared with young HSCs (young: 47 highly proliferative colonies out of 72 total myeloid colonies; aged: 39 out of 52; P = .04). Average colony sizes are indicated by horizontal bars.
The aged HSC pool has a lower frequency of T-competent cells and fewer highly proliferative T-competent clones. (A) Two hundred fifty sorted 2-month-old or 18- to 24-month-old HSCs were plated on OP9-DL1 stromal layers and analyzed on days 17 to 19. Data shown represent 5 independent experiments (*P < .001). (B) Limit-dilution analysis was performed on purified HSCs in vitro by OP9-DL1 coculture. Poisson statistics determined frequencies of lineage-competent cells (T lineage young: 1 in 15 [95% confidence interval 1 in 11-22], aged: 1 in 57 [1 in 33-99], P < .001; myeloid lineage young: 1 in 10 [1 in 7-14], aged: 1 in 14 [1 in 10-19], P = .2). Twenty-four wells were analyzed for each cell dose. (C) Single HSCs were plated on OP9-DL1 layers with cytokines and analyzed by flow cytometry 18 days later. Data shown are restricted to those clones that generated detectable T-lineage progeny and are thus T competent. Data were analyzed using the binomial distribution probability mass function. Colonies with greater than 10 000 progeny were considered highly proliferative (denoted by dashed line), and the frequency of highly proliferative T-competent clones was greater in the young HSC pool than aged (young: 25 highly proliferative colonies out of 50 total T colonies; aged: 10 out of 32; P = .015). Differences were also significant when other arbitrary cutoff values from 5000 to 100 000 were used. For the myeloid lineage, aged HSCs had a higher frequency of highly proliferative myeloid producing clones compared with young HSCs (young: 47 highly proliferative colonies out of 72 total myeloid colonies; aged: 39 out of 52; P = .04). Average colony sizes are indicated by horizontal bars.
We reasoned that production of fewer T-lineage progeny by aged HSCs could be a result of a reduced proliferative burst of committed T-lineage cells and/or a reduction in T-precursor frequency within the HSC pool. To test these possibilities, we performed a limit-dilution analysis of 2-month-old and 18- to 24-month-old HSCs using OP9-DL1 (Figure 5B). Poisson statistical analysis indicated that whereas 1 in 15 young HSCs produced T-lineage cells in this assay, only 1 in 57 aged HSCs did so (P < .001). For myeloid cell production, no significant difference was observed between aged and young HSCs (1 in 14 aged versus 1 in 10 young, P = .2).
To assess burst size of young and aged HSCs during differentiation, single cells were plated onto OP9-DL1, and the number of T and myeloid lineage cells produced per well were counted (Figure 5C). For both young and aged HSCs, there was large variation in T and myeloid output between individual clones. Aged HSCs had more highly proliferative myeloid-competent cells than young HSCs. However, among T-competent clones, a smaller fraction of aged clones were highly proliferative compared with young clones (Figure 5C). Together, these data show that production of fewer T-lineage cells by aged HSCs is the result of both a lower fraction of T-competent cells within the aged HSC pool as well as a smaller T-proliferative burst by T-competent HSCs.
Discussion
In this study, we have shown that BM progenitors from aged mice have defects in T-lineage differentiation. These defects are maintained when aged progenitors are placed in a young host for extended periods of time and are also evident when single aged HSCs are assessed in vitro, indicating that they are cell intrinsic. Multiple cellular mechanisms account for the observed defective T differentiation by aged progenitors. In the HSC pool, there are 2 distinct factors: (1) there is an accumulation of T-compromised cells within the HSC pool; and (2) the HSCs that are T competent make on average fewer T-lineage cells. In contrast, the MPP pool does not exhibit an age-related accumulation of T-compromised cells; however, aged MPPs give rise to fewer T progeny. The simplest interpretation of these data is that T-compromised HSCs do not transition to the downstream MPP pool, and the smaller burst size of T-competent HSCs is also reflected in their MPP progeny. Importantly, we did not detect any reduction in myeloid production by aged progenitors. Hence, the mechanism underlying reduced T output by aged MPPs and the T-competent subset of aged HSCs is not a global defect but instead is linked to defective T or lymphoid lineage differentiation in aged cells.
The loss of T-lineage potential in aged HSCs has historically been difficult to detect, mainly because of the reliance on irradiation models to assay HSC function. It is unclear why age-related changes in T potential are not readily revealed in irradiated mice, but one possible explanation lies in the observation that hematopoiesis in irradiated mice is driven by a small number of stem cell clones, and other clones, while present, remain quiescent.31,32 It is possible that T-compromised HSCs are more likely to be forced into quiescence in the irradiated host, while the competent HSCs drive hematopoiesis. Alternatively, aged T-compromised clones may not engraft in irradiated hosts. Consistently, the engraftment ability of aged HSCs is lower than young HSCs in irradiated hosts.19,33 Another possibility is that transplantation into an irradiated host affects both aged and young HSC function by stimulating rapid proliferation and self-renewal. One could envision a situation where epigenetic control of lymphoid or myeloid gene loci is altered by rapid proliferation.34 Thus, aged/young mixed-marrow radiation chimeras may read out competition between 2 similarly altered populations. A related possibility is that the irradiated environment may produce some limiting lymphopoietic cytokine or chemokine in excess, reactivating aged cells to make them competitive with young cells.35 Importantly, our results here indicate that irradiated mice are not always an appropriate system for assessing hematopoietic function.
It is unclear whether the T-compromised cells that accumulate within the aged HSC pool are true stem cells (ie, have long-term self-renewal ability). Other groups have provided evidence for myeloid-biased or myeloid-restricted HSCs in young mice.36,37 It is possible that the T-compromised cells observed here result from an expansion of such populations with age. Alternatively, the T-compromised cells in the aged HSC pool may not be stem cells at all and instead may be nonrenewing myeloid progenitors. Discrimination between these possibilities will require ways of physically separating the T-competent and T-compromised populations within the “HSC” compartment of aged mice, so that these 2 subsets can be assessed for long-term self-renewal ability in vivo.
Although we clearly demonstrated age-related defects in T-cell production by BM progenitors, it is unknown whether these defects contribute to or initiate thymic involution. Thymic involution is a complex process involving thymic stromal cells and thymocytes, and crosstalk between these 2 populations is required for normal thymus development.38,39 Therefore, defects in 1 compartment can potentially affect the other. On the progenitor side, it is unknown if reductions in MPP number and function described here are limiting for thymopoiesis. The thymus is thought to be periodically settled by progenitors released from the BM,40 so it is possible that decreased MPP function may be compensated for by increased progenitor emigration from the BM. Further, though the MPP pathway is likely important for thymopoiesis,14,41 additional pathways may exist. Several populations, including the CLP-2 in BM and the CTP in BM and blood, also have T potential and may migrate to the thymus.42–44 As these populations have not been assessed in aged mice, it is possible that waning of the MPP pathway is accompanied by an increased role for other pathways. Additionally, these studies focus on the long-lived C57BL/6 mouse strain. However, HSC number and rates of thymus involution vary between mouse strains.45,46 Thus, additional work is needed to determine the contribution of progenitor defects to thymic involution and to establish commonalities of aging mechanisms among different mouse strains and human populations.
Molecular mechanisms underlying defective T differentiation by aged progenitors remain obscure. However, parallels in age-related changes in T lymphopoiesis and B lymphopoiesis, and relative absence of myelopoietic defects, hint that the mechanism may be common to both lymphoid lineages. Like MPPs and ETPs, early B-lineage progenitors are reduced in frequency and number in aged mice.12 Additionally, they are less efficient at producing B-lineage progeny in vitro, which may be a result of reduced responsiveness to IL-7.12,47 Diminished response to IL-7 is an attractive possible mechanism for progenitor aging, as it would be predicted to impact B- and T-lineage differentiation early in development, without affecting myelopoiesis.48
The data presented here resolve the question of whether T-lineage potential of BM progenitors is impacted by aging. We identified T-compromised progenitors within the HSC compartment of aged mice. Further, we showed that aged clones competent for T-lineage differentiation produce fewer T-lineage progeny. Our observations may have important implications for patients who experience profound lymphocyte depletion, usually as a result of chemotherapy or HIV infection. Recovery of T-cell numbers by autologous BM transplantation is impaired with age, and this is correlated with generation of fewer T cells by the thymus.3,4 Although age-related changes in the thymic stroma may play a role in reduced thymic output, our data suggest that age-related changes in BM progenitors are an additional obstacle for efficient production of naive T cells. Additionally, there have recently been efforts to develop effective ex vivo strategies to generate T-lineage cells for patients with impaired thymic function.49–51 Our data point out that the efficacy of these methods may be reduced when using hematopoietic progenitors from aging patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant AI059621. Individual support was provided by NIH training grant T32-CA09140 (V.P.Z.), a Damon Runyon Cancer Research Foundation (I.M.; DRG-102-05), and a Scholar Award from the Leukemia and Lymphoma Society (A.B.). We thank Michael Cancro, David Allman, Warren Pear, Arivazhaghan Sambandam, and Benjamin Schwarz for critically reading the manuscript.
National Institutes of Health
Authorship
Contribution: V.P.Z. designed and performed research, analyzed data, and wrote the paper; I.M. performed research and wrote the paper; and A.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Avinash Bhandoola, 264 John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA 19104; e-mail: bhandooa@mail.med.upenn.edu.

![Figure 4. Aged MPPs are inefficient at producing T-lineage cells in vivo and in vitro. (A) Two thousand sorted MPPs from 2-month-old or 18- to 24-month-old donors were injected into the thymi of nonirradiated recipients, and donor-derived DP progeny were analyzed 21 days later. Error bars represent 1 SEM (*P = .05). (B) Two hundred to 250 purified MPPs were plated onto OP9-DL1 stromal layers supplemented with IL-7 and FL, and numbers of T-lineage (Thy1+ CD25+) and myeloid lineage (Mac1+) progeny were assessed 8 to 10 days later. For bar graphs, error bars represent 1 SEM (*P < .01). (C) Limit-dilution analysis was performed in vitro by culture of purified 2-month-old and 18- to 24-month-old MPPs on OP9-DL1 stromal layers. Poisson statistics determined frequencies of lineage-competent cells (T lineage young: 1 in 19 [95% confidence interval 1 in 13-26], aged: 1 in 21 [1 in 15-29], P = .6; myeloid lineage young: 1 in 13 [1 in 9-17], aged: 1 in 10 [1 in 8-14], P = .3). Twenty-eight wells were analyzed for each cell dose.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2007-01-071605/5/m_zh80150705530004.jpeg?Expires=1769275988&Signature=kaBoXUhrNz~WmEaJUOs2nv7c3Xij8m~55gnnSf8Xu8xCOBviJXlQ2oPjHpUi~MNndBTEJn1fwpH~QUo0-defZVHOG7gMVnERwT9TU9ZXbDvTEw5ra0wgRs645qj~Yvk9M1vCGOlbTnZVJLVUGaUkYtwO3MX~oOZo9QpNi6EDHUh4IFSJc3x8sdF7dK1mHhlIl5Bfonl2GY3~XKVVOPYQKQIr7DVa50d5r6tB9MYL2tkGOD63Zmkqh1OMWYf7T6xFWgLO3K6a~BNbYMb2mTQo2LIEPod7YgRwu8Hppaood17zT8WDt7i0qHKjTgL7aemUius7GpUKkPgFm1A8Xfnw5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. The aged HSC pool has a lower frequency of T-competent cells and fewer highly proliferative T-competent clones. (A) Two hundred fifty sorted 2-month-old or 18- to 24-month-old HSCs were plated on OP9-DL1 stromal layers and analyzed on days 17 to 19. Data shown represent 5 independent experiments (*P < .001). (B) Limit-dilution analysis was performed on purified HSCs in vitro by OP9-DL1 coculture. Poisson statistics determined frequencies of lineage-competent cells (T lineage young: 1 in 15 [95% confidence interval 1 in 11-22], aged: 1 in 57 [1 in 33-99], P < .001; myeloid lineage young: 1 in 10 [1 in 7-14], aged: 1 in 14 [1 in 10-19], P = .2). Twenty-four wells were analyzed for each cell dose. (C) Single HSCs were plated on OP9-DL1 layers with cytokines and analyzed by flow cytometry 18 days later. Data shown are restricted to those clones that generated detectable T-lineage progeny and are thus T competent. Data were analyzed using the binomial distribution probability mass function. Colonies with greater than 10 000 progeny were considered highly proliferative (denoted by dashed line), and the frequency of highly proliferative T-competent clones was greater in the young HSC pool than aged (young: 25 highly proliferative colonies out of 50 total T colonies; aged: 10 out of 32; P = .015). Differences were also significant when other arbitrary cutoff values from 5000 to 100 000 were used. For the myeloid lineage, aged HSCs had a higher frequency of highly proliferative myeloid producing clones compared with young HSCs (young: 47 highly proliferative colonies out of 72 total myeloid colonies; aged: 39 out of 52; P = .04). Average colony sizes are indicated by horizontal bars.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2007-01-071605/5/m_zh80150705530005.jpeg?Expires=1769275988&Signature=Eu-cpqN-QYH9LwyuVYCEjtzXBZ5aCG-tu71rG0YEv4Nt1K7vHUvKrSuiEzvVZQ6wujhYgQW6PxTipviZAVyeGSUgWnUL0oQ2JztaB5CtBVF8VfI~1HaFpkvtBd-K8y3ic33RVoPUOYoberFzxP~kJMw4~DconAzqbpD1axW4p46u~TkjobEc9MnTK9qU7QKo~kBF-eNj1BIQp9ZFptePeWFezF2C-CZHaM-tNZQV2io76exXr0AOzUvTVh3a0Wb4pb9q1ukuB-TNMRInZ9x9OIlNVIxq7DC8nYf6kDrdLWFBycdg9omke8am9d6vJFkrf0q~7Cf~78F6KMM0A~2fhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
