Abstract
Recombinant osteoprotegerin (OPG) promoted the adhesion of both primary polymorphonuclear neutrophils (PMNs) and leukemic HL60 cells to endothelial cells. Leukocyte/endothelial cell adhesion was promoted by short (peak at 1 hour) preincubation of either endothelial cells or PMNs with OPG, and the peak of proadhesive activity was observed in the same range of OPG concentrations detected in the sera of patients affected by cardiovascular diseases. Although the cognate high-affinity ligands for OPG, membrane receptor activator of nuclear factor-κB ligand (RANKL) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), were detected at significant levels on both PMNs and HL60 cells, they were not expressed on the surface of endothelial cells. However, preincubation of OPG with heparin abrogated its proadhesive activity, whereas pretreatment of endothelial cells with chondroitinase plus heparinases significantly decreased the proadhesive activity of OPG. Taken together, these findings suggest the involvement of both the ligand binding and the N-terminal heparin-binding domains of OPG in mediating its pro-adhesive activity. The relevance of these in vitro findings was underscored by in vivo experiments, in which the topical administration of recombinant OPG increased leukocyte rolling and adhesion to rat mesenteric postcapillary venules. Our data suggest that a pathological increase of OPG serum levels might play an important role in promoting leukocyte/endothelial cell adhesion.
Introduction
Osteoprotegerin (OPG), a soluble member of the tumor necrosis factor (TNF) receptor superfamily, was originally characterized for its ability to suppress osteoclast formation.1 OPG inhibits osteoclastogenesis by binding to receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL), a member of the TNF superfamily of cytokines, and preventing the interaction of RANKL with its high-affinity transmembrane receptor (receptor activator of NF-κB [RANK]).2 It has been shown that in the low nanomolar range, the binding interaction of OPG and RANKL is 1:13 and that dimerization of OPG results from noncovalent interactions mediated by the death domains and, to a lesser extent, by a C-terminal heparin-binding region. OPG dimer formation is required for the mechanism of inhibition of the RANKL/RANK receptor interaction.3
OPG can also interact with another member of the TNF superfamily, TNF-related apoptosis inducing ligand (TRAIL),4 which can kill a variety of cancer cell types both in vivo and in vitro.5 A role for OPG as a neutralizing receptor for TRAIL under physiological conditions has been questioned in early studies, but mounting evidence now suggests that the OPG/TRAIL interaction is biologically important, at least in in vitro culture systems.6 OPG has been shown to act in a paracrine or autocrine manner by binding TRAIL and promoting the survival of prostate cancer cells,7 breast cancer cells,8 and multiple myeloma cells.9 Moreover, when a rationally designed small molecule mimic of OPG was examined for association with TRAIL or RANKL in binding studies, this peptide bound to RANKL at a Kd of 3.89 × 10−6 M and to TRAIL at a Kd of 1.93 × 10−5 M, showing only about 5-fold higher affinity for recombinant RANKL compared with recombinant TRAIL.10
A number of studies have clearly demonstrated that the serum levels of OPG are elevated in both patients with diabetes and those without who are affected by coronary artery disease and heart failure after acute myocardial infarction, and increased levels of OPG in these patients represent a risk factor for cardiovascular mortality.11-18 OPG serum levels are also elevated in patients affected by a variety of human malignancies, including hematological disorders,19,20 and an immunohistochemical study has demonstrated that OPG is expressed in the tumor-associated endothelium in approximately 60% of malignant tumors.21
Despite the reported findings, the physiopathological role of elevated serum levels of OPG in vascular biology is not well understood. On these bases, we have investigated the effect of OPG on the endothelial-leukocyte interactions in both in vitro and in vivo experimental models.
Materials and methods
All the animal experimental procedures were performed in compliance with the guidelines of European (86/609/EEC) and the Italian (D.L.116/92) laws and approved by the Italian Ministry of University and Research as well as by the Administration of the Animal House of the University of Trieste. Human blood samples were obtained after informed consent and after full explanation of the procedure and its purpose, in accordance with Declaration of Helsinki of 1975, and with approval from the local (University of Ferrara) institutional review boards.
Cells and reagents
Primary human umbilical vascular endothelial cells (HUVECs) and human microvascular endothelial cells (HMVECs) were obtained from Lonza Walkersville Inc (Walkersville, MD). HUVECs and HMVECs were used between the second and fifth passage in vitro and were cultured in EGM basal medium supplemented with 2% fetal bovine serum (FBS), 12 mg/mL bovine brain extract (BBE), 1 mg/mL hydrocortisone, and 10 ng/mL endothelial cell growth factor (ECGF) (all from Lonza), as described previously.22,23
EDTA (ethylenediaminetetraacetic acid)-blood samples for the isolation of primary polymorphonuclear neutrophils (PMNs) were drawn from healthy volunteers after obtaining informed consent. Blood samples were diluted 1:2 with phosphate-buffered sodium chloride solution (PBS), and a first separation step was performed by Lymphoprep (Axis-Shield PoC, Oslo, Norway). PMNs in the pellet of the gradient were separated from erythrocytes by further steps consisting of dextran sedimentation and hypotonic lysis, resulting in purity of less than 90% CD15/CD11b+ cells, as assessed by flow cytometry using specific fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs; Immunotech, Marseille, France; Miltenyi Biotech GmbH, Bergish Gladbach, Germany). Treatments were performed immediately after cell isolation. The HL60 myeloid cell line (American Type Culture Collection, Manassas, VA) was routinely grown in RPMI 1640 medium (Invitrogen, Paisley, United Kingdom) supplemented with 10% FBS.
Heparin, heparinases I, II, and III, and chondroitinase ABC were purchased from Sigma Chemicals (St Louis, MO). TNF-α and recombinant OPG were from R&D Systems (Minneapolis, MN). In particular, the recombinant full-length OPG (Met1-Leu401) was expressed in the NSO mouse myeloma cell line. After purification, the recombinant OPG, a disulfide-linked homodimeric protein generated after removal of a 21-amino acid signal peptide from each monomer, is lyophilized from a 0.2-μm filtered solution in PBS (“carrier free”). In selected experiments, we have also used the recombinant OPG-Fc, corresponding to the cysteine-rich region of human OPG (amino acids 22-202) fused to the Fc portion of human IgG1, therefore lacking the N-terminal heparin-binding domain.
Human OPG levels were measured in HUVEC culture supernatants using sandwich-type enzyme-linked immunosorbent assay (ELISA) kit (Alexis Biochemicals, Lausen, Switzerland), according to the manufacturer's instructions. The results were read using an Anthos 2010 ELISA reader (Anthos Labtec Instruments GmbH, Wals, Austria). Measurements were done in duplicate.
Endothelial-leukocyte adhesion assay
Vascular endothelial cells were grown to confluence in 24- or 96-well tissue culture plates and stimulated for 1 minute to up to 16 hours (overnight) at 37°C with recombinant human OPG or TNF-α, used alone or in combination. After 3 washings with serum-free medium, untreated up to 3.5 × 105 PMNs or HL60 cell suspensions were added to each well and were further incubated at 37°C for 60 minutes. In some experiments, leukocytes were added simultaneously with recombinant OPG to HUVECs for up to 60 minutes. After endothelial-leukocyte coculture, nonadherent PMNs or HL60 cells were removed by washing the wells at least twice. Endothelial-leukocyte cocultures were photographed under a light microscope (10× magnification). The number of adhered PMNs or HL60 cells was evaluated by a colorimetric assay using tetramethyl benzidine (Sigma Chemicals) as a substrate for myeloperoxidase, as described previously,24 and/or by scoring at least 6 random fields for each treatment. In both assays, the viability of both endothelial cells and adherent PMNs or HL60 cells was routinely monitored at light microscopy by Trypan blue dye exclusion.
In some experiments, recombinant OPG was preincubated with heparin (250 mg/mL) for 1 hour at 37°C before performing adhesion assay. In other experiments, HUVEC cultures were pretreated with heparinases I, II, and III (50 U/mL each), a family of enzymes that cleaves highly sulfated regions of heparin sulfate-like glycosaminoglycans (GAGs) at 2-0 sulfated uronic acids, and with chondroitinase ABC (50 U/mL), which cleaves the chondroitin sulfate side chain of cell-surface GAGs,25 for 1 hour at 37°C. After washing, adhesion experiments were performed.
Expression of adhesion molecules
Total RNA was extracted from HUVECs either left untreated or stimulated with recombinant OPG or TNF-α by using the QIAGEN RNeasy mini kit (QIAGEN, Hilden, Germany) according to the supplier's instructions. The quality of the total RNA preparation was verified by agarose gel and, when necessary, further purification was performed with the RNeasy cleanup system (QIAGEN) to remove chromatin DNA. Amplification for adhesion molecules was performed with a real-time polymerase chain reaction (PCR) system (model 7500; Applied Biosystems, Foster City, CA) using SYBR Green based-technology and the RT2 Real-Time Gene Expression Assays (SuperArray Bioscience Corporation, Frederick, MD), that include specific validated primer sets and PCR master mixes (SuperArray Bioscience Corporation). All samples were run in triplicate.
In parallel experiments, E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) expression in HUVEC cultures was determined by ELISA. In brief, cells were washed with PBS, 2% BSA, and 0.7 mM Ca2+Mg2+ before incubation with 5 μg/mL of the following MoAb: anti-ICAM-1 (Dako Denmark A/S, Glostrup, Denmark), anti-VCAM-1 and anti-E-selectin (both from Chemicon, Temecula, CA), and then with APC-anti-mouse secondary antibody (Sigma Chemical). Finally p-nitrophenyl phosphate (PNPP; Sigma Chemicals) was applied to cells, and the absorbance was measured at 405 nm by an ELISA reader.
Flow cytometric analyses
Expression of surface cellular antigens on HL60, PMNs, and/or HUVECs was evaluated by flow cytometric analysis with the FACScan (BD Biosciences, San José, CA). Surface cell staining was performed at 4°C for 40 minutes by incubating 3 to 5 × 105 cells in 200 μl of PBS (containing 1% BSA and 5% FBS) with 1 μg the indicated antibodies (Abs). In particular, the expression of surface TRAIL and RANKL was evaluated by indirect staining with primary Abs (both from R&D Systems), followed by PE-conjugated anti-mouse secondary Ab (Immunotech). Nonspecific fluorescence was assessed using normal mouse isotype-match IgG followed by secondary Abs. On the other hand, mAbs directly conjugated with FITC or PE were used for the detection of CD15 (Immunotech) and Mac-1 (Miltenyi) on PMNs or HL60 cells, as well as for the detection of E-selectin, ICAM-1 (both MoAbs from Bender Medical System, Wien, Austria), and VCAM-1 (Cymbus Biotechnology, Chandlers Ford, United Kingdom). Nonspecific fluorescence was assessed by incubation with irrelevant isotype-matched conjugated MoAbs.
In other experiments, PMNs, HL-60 cells, or HUVECs were incubated with either recombinant OPG or OPG-Fc for 40 minutes at 37°C, and after washing, the adhesion of OPG or OPG-Fc to the cell surface was revealed by incubation with MoAb anti-human OPG (R&D Systems) followed by PE-conjugated anti-mouse secondary Abs.
Animals and in vivo experimental procedure
Male Wistar Kyoto rats, each weighting approximately 250-270 g, were anesthetized intraperitoneally with sodium thiobarbital (100 mg/kg). Details on the surgical procedure, including insertion of intravascular catheters to monitor blood pressure and to inject the fluorescent leukocyte marker acridine orange (AO) were reported previously.24 AO was diluted in sterile sodium chloride solution and then slowly infused during the experimental procedure at the concentration of 0.025 mg/kg/min and at a rate of 0.5 mL/h. The rats were placed on an adjustable stage of an upright microscope (model BX50WI; Olympus Optical, Tokyo, Japan; immersion objective model UM Plan FL Olympus, magnification 10×, numerical aperture 0.30W), and after a midline incision of the rat abdomen, a loop of ileal mesentery was exteriorized and carefully draped over a transparent pedestal. The exposed tissue was infused throughout the study with sterile buffered sodium chloride solution (Krebs-Henseleit modified solution: NaCl 118 mM, KCl 4.74 mM, CaCl 2.45 mM, KH2PO4 1.19 mM Mg SO4 1.19 mM, NaHCO3 12.5 mM) warmed at 37°C. The AO-labeled leukocytes were made visible by epifluorescence transillumination with a UIF550 filter for excitation light (Olympus Optical, Tokyo, Japan). Images were recorded by charge-coupled device camera (SensiCam PCO, Kelheim, Germany) connected through a PCI interface board (SensiCam digital converter) to a computer device, where they were stored and analyzed off-line using dedicated imaging software (Analytica Lite, Milan, Italy).
After surgery and a baseline evaluation, sterile sodium chloride solution containing recombinant OPG was topically applied to the mesentery, and after 4 hours, image sequences were recorded intermittently at different time intervals up to 90 minutes (control animals were treated with the vehicle). Segments of 3 to 5 unbranched postcapillary venules (25- to 40-μm diameter, 200-μm length) were selected for analysis, as described previously.24 Venular diameter and center-line red blood cell velocity were evaluated off-line using a video caliper (Image Research, Ontario, Canada) and customized frame-by-frame analogical image analysis. Red blood cell velocity (VRBC) and venular diameter (D) were used to calculate venular wall shear rate (g) through the formula g = 8(Vmean/D), where V mean = VRBC/1.6. Intravascular circulation of leukocytes in postcapillary venules was analyzed off-line during playback of the digital file sequences. Labeled leukocytes were classified as rolling (if they moved more slowly than red blood cells, thus becoming visible) or adherent (if they remained stationary for more than 30 seconds). Rolling flux was expressed as the number of leukocytes seen moving past a reference point per minute, and adherence was measured by counting the number of adherent leukocytes per 200 μm of venule length.
Statistical analysis
The results were evaluated by using analysis of variance with comparison by Student's t test and with the Mann-Whitney rank-sum test. Statistical significance was defined as P is less than .05.
Results
Recombinant OPG promoted the leukocyte/endothelial cell adhesion
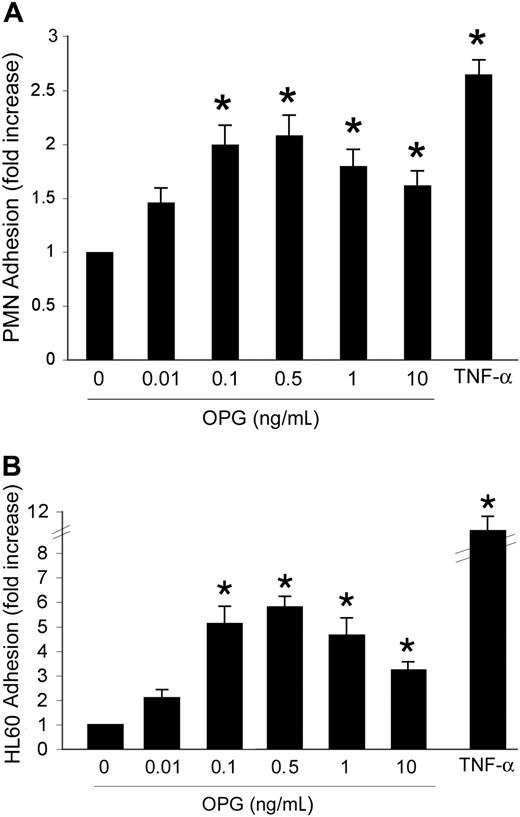
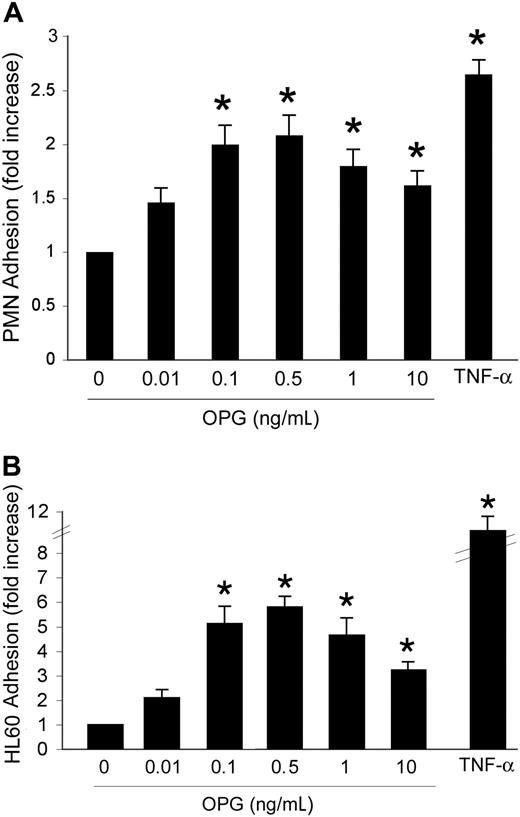
Our initial experiments were designed to investigate whether OPG might affect leukocyte/endothelial interactions. HUVECs were grown to confluence and incubated with increasing concentrations (0.01-10 ng/mL) of recombinant human OPG. In parallel, cells were treated with TNF-α (0.1 ng/mL), used as positive control. An overnight incubation (approximately 16 hours) of HUVECs with recombinant OPG induced a bell-shaped increase in the number of adherent PMNs with respect to HUVECs left untreated (Figure 1A). It is noteworthy that OPG showed maximal proadhesive activity at concentrations between 0.1 and 0.5 ng/mL, which are in the range of concentrations reported to be elevated in the sera of patients affected by cardiovascular diseases.11-18 Similar findings were obtained when experiments were carried out using HMVECs instead of HUVECs (data not shown), indicating that the OPG proadhesive activity was not endothelial cell type-specific. In parallel experiments, another cell system was used to test whether the OPG effects were reproducible and could be generalized. For this purpose, we chose a HL60 myeloid leukemic cell line, taking into account that serum OPG has been reported to be elevated also in the sera of patients affected by hematological malignancies.19 OPG induced a significant (P > .05) bell-shaped increase of HL60 leukemic cell adhesion to HUVECs, starting from concentrations as low as 0.1 ng/mL (Figure 1B).
Dose-response effect of OPG on leukocyte adhesion. HUVEC cells were either left untreated or exposed for 16 hours to the indicated concentrations of OPG. TNF-α (0.1 ng/mL) was used as positive control. After HUVEC washing, (A) PMNs or (B) HL-60 cells were added to the endothelial monolayer, and the percentage of adherent cells was determined after a coculture of 60 minutes at 37°C. Cell adherence on HUVECs is reported as fold of increase with respect to cell adhesion in the absence of treatment. Results are expressed as mean ± SD of 7 experiments, each performed in triplicate. ∗, P > .05.
Dose-response effect of OPG on leukocyte adhesion. HUVEC cells were either left untreated or exposed for 16 hours to the indicated concentrations of OPG. TNF-α (0.1 ng/mL) was used as positive control. After HUVEC washing, (A) PMNs or (B) HL-60 cells were added to the endothelial monolayer, and the percentage of adherent cells was determined after a coculture of 60 minutes at 37°C. Cell adherence on HUVECs is reported as fold of increase with respect to cell adhesion in the absence of treatment. Results are expressed as mean ± SD of 7 experiments, each performed in triplicate. ∗, P > .05.
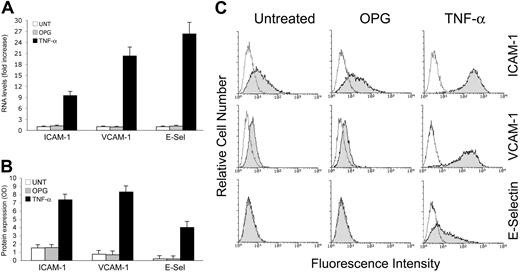
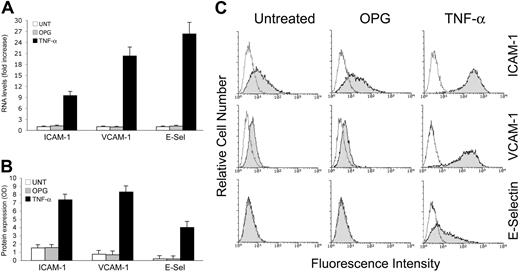
Proinflammatory cytokines, such as TNF-α, are known to increase leukocyte adhesion to endothelial cells through several mechanisms, including the up-regulation of surface adhesion molecules, such as ICAM-1, VCAM-1 and E-selectin, in endothelial cells at the mRNA level (Figure 2A), and at the protein level (Figure 2B,C). On the contrary, recombinant OPG did not induce any significant increase of ICAM-1, VCAM-1 or E-selectin mRNA (Figure 2A). Consistently with the mRNA data, OPG was unable to modulate ICAM-1, VCAM-1, or E-selectin at the protein level, as evaluated by 2 independent approaches: ELISA (Figure 2B) and flow cytometry (Figure 2C), which allowed us to quantify the total and surface expression levels of adhesion molecules, respectively.
Lack of effect of OPG on the expression of adhesion molecules. HUVECs were exposed to OPG or TNF-α (used as positive control) for 16 hours and expression levels of adhesion molecules (ICAM-1, VCAM-1, and E-selectin) were evaluated at both (A) mRNA and (B) protein levels by ELISA and (C) flow cytometry. (A) RNA from HUVECs either left untreated or exposed to OPG or TNF-α were quantitatively analyzed by ICAM-1, VCAM-1 and E-selectin RT-PCR after normalization to the level of GAPDH mRNA. Each sample was determined in duplicate. (B) Adhesion molecules expression was determined by ELISA and data are shown as average OD ± SD of 3 independent experiments. In panel C, the control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control antibodies. One of 6 experiments with similar results is shown.
Lack of effect of OPG on the expression of adhesion molecules. HUVECs were exposed to OPG or TNF-α (used as positive control) for 16 hours and expression levels of adhesion molecules (ICAM-1, VCAM-1, and E-selectin) were evaluated at both (A) mRNA and (B) protein levels by ELISA and (C) flow cytometry. (A) RNA from HUVECs either left untreated or exposed to OPG or TNF-α were quantitatively analyzed by ICAM-1, VCAM-1 and E-selectin RT-PCR after normalization to the level of GAPDH mRNA. Each sample was determined in duplicate. (B) Adhesion molecules expression was determined by ELISA and data are shown as average OD ± SD of 3 independent experiments. In panel C, the control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control antibodies. One of 6 experiments with similar results is shown.
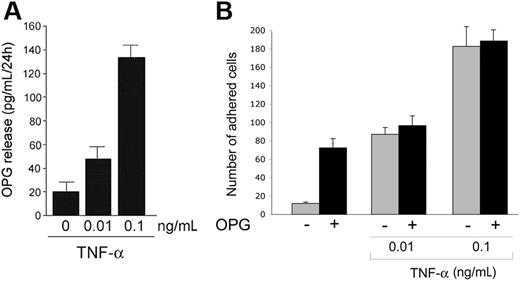
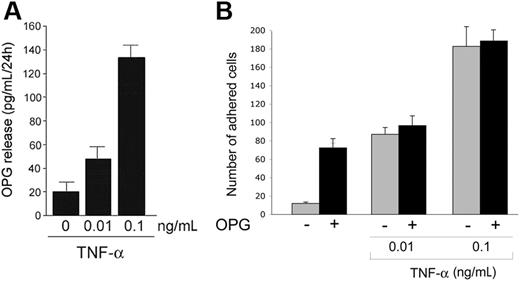
As shown in Figure 3A, whereas untreated HUVECs released low levels of OPG in the culture supernatants, TNF-α induced a dose-dependent increase of the release of endogenous OPG by HUVECs. Interestingly, the levels of OPG released in response to TNF-α were in the range of OPG concentrations able to promote binding of both PMNs and HL60 cells to endothelial cells (Figure 1), suggesting the possibility that OPG endogenously released in response to TNF-α might contribute to TNF-α-mediated proadhesive activity. Moreover, the simultaneous overnight treatment of HUVECs with recombinant OPG (0.5 ng/mL) plus TNF-α (0.01-0.1 ng/mL) showed no additive or synergistic effects with respect to the pro-adhesive activity induced by TNF-α alone (Figure 3B).
Endogenous OPG release by endothelial cells. (A) HUVECs were either left untreated or stimulated with the indicated concentration of TNF-α. After 24 hours, the levels of OPG released in culture supernatant were measured by ELISA. Results are expressed as means ± SD of 4 independent experiments, each performed in triplicate. (B) HUVECs were either left untreated or stimulated with OPG (0.5 ng/mL) in the presence or absence of TNF-α. After washing, leukocytes were added to endothelial monolayer, and the number of adherent leukocytes was scored. Values are mean ± SD of triplicate determinations of 3 separate experiments.
Endogenous OPG release by endothelial cells. (A) HUVECs were either left untreated or stimulated with the indicated concentration of TNF-α. After 24 hours, the levels of OPG released in culture supernatant were measured by ELISA. Results are expressed as means ± SD of 4 independent experiments, each performed in triplicate. (B) HUVECs were either left untreated or stimulated with OPG (0.5 ng/mL) in the presence or absence of TNF-α. After washing, leukocytes were added to endothelial monolayer, and the number of adherent leukocytes was scored. Values are mean ± SD of triplicate determinations of 3 separate experiments.
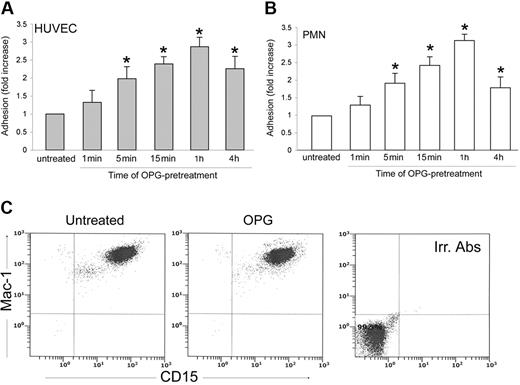
A short-term exposure of either endothelial cells or PMNs to recombinant OPG was sufficient to promote leukocyte/endothelial adhesion
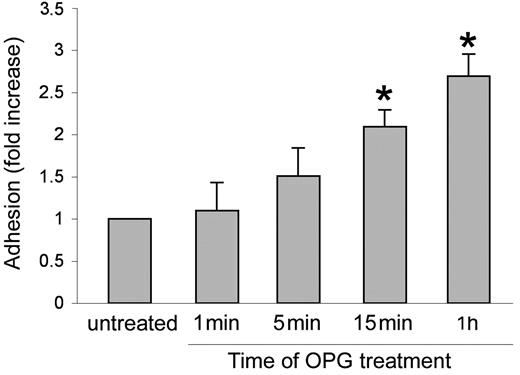
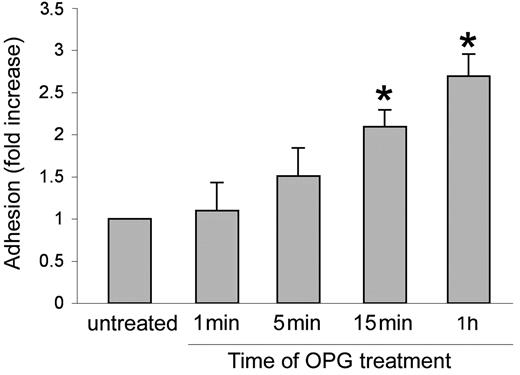
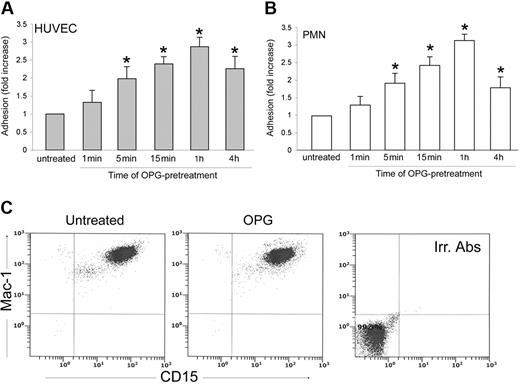
In the next series of experiments, an optimal concentration of recombinant OPG (0.5 ng/mL) was added simultaneously with PMNs to endothelial cells for up to 1 hour (Figure 4). The presence of recombinant OPG induced a significant (P > .05) increase of leukocyte adhesion to endothelial cells as early as 15 minutes after the beginning of PMN/endothelial cell cocultures. This rapid induction of proadhesive activity suggested that transcriptional-independent mechanisms should account for this biological activity of OPG. To further characterize the adhesive activity of OPG, endothelial cells were pretreated with recombinant OPG (0.5 ng/mL) for different time points, washed 3 times to eliminate unbound OPG, and then co-incubated with PMNs (Figure 5A). In parallel, PMNs, instead of endothelial cells, were pretreated with recombinant OPG (0.5 ng/mL) for different time points, abundantly washed and then co-incubated with endothelial cells (Figure 5B). As shown in Figure 5A, a significant (P > .05) increase of the proadhesive activity of OPG was observed for times of HUVECs pretreatment as short as 5 minutes. In addition, a short pretreatment of PMNs with OPG significantly (P > .05) increased their adhesiveness to HUVECs (Figure 5B), at a comparable extent and kinetics with respect to the pro-adhesive activity observed in experiments in which HUVECs were pretreated with OPG before coculture with PMNs. Moreover, exposure of PMNs to recombinant OPG for up to 4 hours induced no modulation of the surface expression levels of Mac-1 (Figure 5C), which was investigated taking into account that such leukocyte surface molecules play a pivotal role for binding to ICAMs and mediating leukocyte extravasation.26
Effect of short-term OPG treatment on leukocyte/endothelial cell adhesion. Endothelial monolayers were exposed to PMNs in the absence or presence of recombinant OPG (0.5 ng/mL) for the indicated times before scoring the number of adherent PMNs. Cell adhesion in untreated control culture was set as unity. Values are mean ± SD of triplicate determinations of 3 separate experiments. ∗, P > .05 versus control.
Effect of short-term OPG treatment on leukocyte/endothelial cell adhesion. Endothelial monolayers were exposed to PMNs in the absence or presence of recombinant OPG (0.5 ng/mL) for the indicated times before scoring the number of adherent PMNs. Cell adhesion in untreated control culture was set as unity. Values are mean ± SD of triplicate determinations of 3 separate experiments. ∗, P > .05 versus control.
Effect of OPG pretreatment on leukocyte/endothelial cell adhesion and determination of Mac-1 expression in PMNs. (A) Endothelial monolayers or (B) PMNs were either left untreated or exposed to OPG (0.5 ng/mL) for the indicated times. After washing, PMNs and endothelial cells were cocultured for 1 hour, and the number of adherent PMNs was scored. Cell adhesion in untreated control culture was set as unity. Values are mean ± SD of triplicate determinations of 5 separate experiments. ∗, P > .05 versus control. (C) PMNs were either left untreated or exposed to OPG (0.5 ng/mL), and surface expression levels of Mac-1 on CD15+ cells was evaluated by double staining in flow cytometry. Irr. Abs: staining of the PMN culture with isotype-matched control antibodies. One of 3 experiments with similar results is shown.
Effect of OPG pretreatment on leukocyte/endothelial cell adhesion and determination of Mac-1 expression in PMNs. (A) Endothelial monolayers or (B) PMNs were either left untreated or exposed to OPG (0.5 ng/mL) for the indicated times. After washing, PMNs and endothelial cells were cocultured for 1 hour, and the number of adherent PMNs was scored. Cell adhesion in untreated control culture was set as unity. Values are mean ± SD of triplicate determinations of 5 separate experiments. ∗, P > .05 versus control. (C) PMNs were either left untreated or exposed to OPG (0.5 ng/mL), and surface expression levels of Mac-1 on CD15+ cells was evaluated by double staining in flow cytometry. Irr. Abs: staining of the PMN culture with isotype-matched control antibodies. One of 3 experiments with similar results is shown.
The heparin-binding domain of OPG was involved in mediating the proadhesive activity of OPG
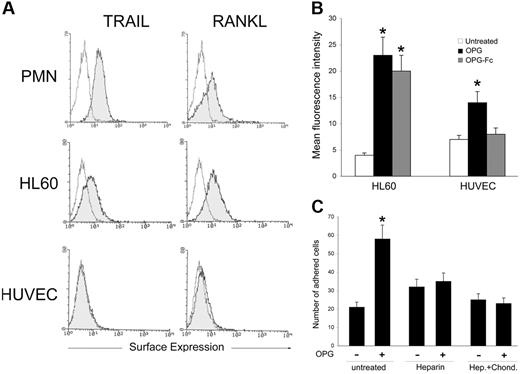
We next explored the possibility that OPG might interact with membrane-associated molecules present on the cell surface of leukocytes as well as on endothelial cells. For this purpose, PMNs, HL60 cells, and endothelial cells were analyzed by flow cytometry for the expression of the cognate high-affinity receptors of OPG, RANKL, or TRAIL, which exist not only as soluble proteins but also as transmembrane proteins.3,5 It is noteworthy that both PMNs and HL60 cells showed a clear-cut expression of both ligands, whereas endothelial cells expressed neither RANKL nor TRAIL (Figure 6A). In parallel, the ability of the full-length OPG and of the truncated OPG-Fc to bind to the surface of leukocytes or endothelial cells was assessed by flow cytometry. As shown in Figure 6B, a significant (P > .01) binding of both OPG and OPG-Fc was measured on leukocytes, as expected based on the presence of both of its high-affinity receptors. It is noteworthy that a modest (P > .05) binding of full-length recombinant OPG was observed also on endothelial cells, whereas recombinant truncated OPG-Fc failed to bind to HUVECs (Figure 6B). This observation suggests that other domains of OPG besides the ligand-binding domain are involved in the OPG/HUVEC interaction, and therefore in mediating the pro-adhesive activity of OPG. In this respect, previous studies have demonstrated that OPG contains 3 structural domains specifically influencing its biological activity.3,27,28 The first one is a cysteine-rich domain in the N-terminal position that is not necessary for OPG dimerization. The second domain is a heparin-binding domain potentially capable of interacting with numerous proteoglycans,28,29 and the third one corresponds to a death domain homologous region.27 To evaluate whether the heparin-binding domain, which is deleted in the OPG-Fc recombinant molecule, might indeed be involved in mediating the proadhesive activity of OPG, recombinant OPG was preincubated with heparin for 40 minutes before either HUVEC or PMN treatment for 1 additional hour. As shown in Figure 6C, a significant (P > .05) decrease in the proadhesive activity of OPG was observed when recombinant OPG was preincubated with heparin. A similar decrease of the proadhesive activity of OPG was observed when HUVECs were pretreated with heparinases plus chondroitinase before exposure to recombinant OPG (Figure 6C). We have also attempted to compare the pro-adhesive activity of full-length recombinant OPG with that of OPG-Fc to further characterize the involvement of the heparin-binding domain in mediating the pro-adhesive activity of OPG. Unfortunately, however, both OPG-Fc and human IgG, used as control, induced a significant increase of leukocyte adhesion to endothelial cells through the rapid activation of Mac-1 in PMNs due to the Fc-moiety (data not shown).
Cell surface binding of recombinant OPG. (A) surface expression of TRAIL and RANKL in PMNs, HL60 cells, and HUVECs was evaluated by flow cytometry. The control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control antibodies. One of 4 experiments with similar results is shown. (B) HL60 and HUVECs were incubated with either recombinant OPG or OPG-Fc, and binding to cell surface was revealed by flow cytometry. Results are expressed as mean fluorescence intensity and are mean ± SD of determinations of 3 separate experiments. (C) The specific contribution of heparan sulfate proteoglycans in OPG-mediated leukocyte adhesion was evaluated by pretreatment of OPG with heparin or pretreatment of HUVECs with heparinase I, II, and III (Hep.) plus Chondroitinase (Chond.) before adhesion assays. Values are mean ± SD of triplicate determinations of 3 separate experiments. ∗, P > .05.
Cell surface binding of recombinant OPG. (A) surface expression of TRAIL and RANKL in PMNs, HL60 cells, and HUVECs was evaluated by flow cytometry. The control (unshadowed) histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control antibodies. One of 4 experiments with similar results is shown. (B) HL60 and HUVECs were incubated with either recombinant OPG or OPG-Fc, and binding to cell surface was revealed by flow cytometry. Results are expressed as mean fluorescence intensity and are mean ± SD of determinations of 3 separate experiments. (C) The specific contribution of heparan sulfate proteoglycans in OPG-mediated leukocyte adhesion was evaluated by pretreatment of OPG with heparin or pretreatment of HUVECs with heparinase I, II, and III (Hep.) plus Chondroitinase (Chond.) before adhesion assays. Values are mean ± SD of triplicate determinations of 3 separate experiments. ∗, P > .05.
Leukocytes adhere to endothelium in vivo in response to OPG
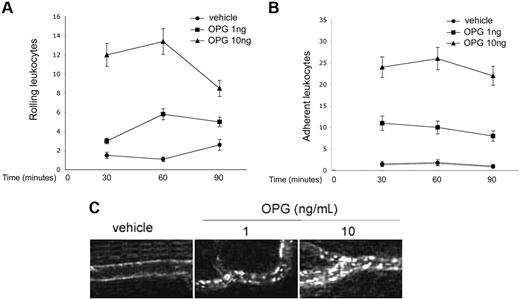
In the last group of experiments, we sought to determine whether OPG was able to promote leukocyte adhesion to endothelial cells also in vivo. Recombinant OPG (1-10 ng/mL) was topically applied to the rat mesentery, and after 4 hours, the rolling and adhesion of leukocytes was determined by intravital microscopy at different time points (30, 60, and 90 minutes). This is a previously established in vivo procedure through which single polypeptides as well as big protein complexes easily enter in the blood circulation through the highly permeable postcapillary venules of the rat mesentery.24 As shown in Figure 7A, leukocyte rolling was significantly (P > .05) and dose-dependently induced by the treatment with OPG with respect to treatment with the vehicle. It is noteworthy as well that the number of leukocytes that adhered to the vessels was significantly (P > .05) higher in rats treated with OPG than in rats receiving saline vehicle (Figure 7B,C). The observation that only a few cells adhered to endothelial cells in control rats treated with sodium chloride solution excludes the possibility that the proadhesive property acquired by the endothelium represents a reaction to the surgical procedure and also rules out nonspecific effects of the fluorescent dye injected intravenously. Moreover, OPG administration induced no modification in the systemic leukocyte and neutrophil counts (data not shown).
Effect of OPG on leukocyte trafficking in rat mesenteric postcapillary venules. Traffic of leukocytes was monitored at various time intervals after 4 hours of intraperitoneal administration of 1-10 ng/mL of OPG, and of the control vehicle. Measurements were performed on 4 to 6 different segments of unbranched venules (25- to 40-μm diameter, 200-μm length). (A) Values of rolling leukocyte flux defined as number of cells that become visible as bright spheres if they travel inside the venules more slowly than red blood cells. (B) Numbers of leukocytes stably adherent to the same site of postcapillary vascular endothelium of rat mesentery. (C) Leukocytes labeled in vivo with AO were made visible by fluorescence epi-illumination and appeared as bright spheres. One of 4 experiments with similar results is shown. Magnification, 100×.
Effect of OPG on leukocyte trafficking in rat mesenteric postcapillary venules. Traffic of leukocytes was monitored at various time intervals after 4 hours of intraperitoneal administration of 1-10 ng/mL of OPG, and of the control vehicle. Measurements were performed on 4 to 6 different segments of unbranched venules (25- to 40-μm diameter, 200-μm length). (A) Values of rolling leukocyte flux defined as number of cells that become visible as bright spheres if they travel inside the venules more slowly than red blood cells. (B) Numbers of leukocytes stably adherent to the same site of postcapillary vascular endothelium of rat mesentery. (C) Leukocytes labeled in vivo with AO were made visible by fluorescence epi-illumination and appeared as bright spheres. One of 4 experiments with similar results is shown. Magnification, 100×.
Discussion
OPG has been shown to be abundantly expressed in vascular cells and in particular in vascular smooth muscle cells both in vitro and in vivo.30-32 Moreover, several studies have clearly demonstrated that OPG plays a role in promoting in vitro endothelial and vascular smooth muscle cell (VSMC) survival and preventing vessel wall calcification.33,36 In fact, mice lacking OPG show calcified arteries,34,35 and an elegant study has recently demonstrated that OPG is strongly expressed in and around the ventricular-side endothelium but it shows a little expression on the aortic side, which is more prone to calcification.36 Despite these findings, which clearly suggest a protective role of physiological concentrations of OPG in the vascular system, other studies have demonstrated that the expression and release of OPG by endothelial cells and VSMCs is markedly up-regulated in response to inflammatory cytokines, such as TNF-α31 and platelet-derived growth factor (PDGF),30 2 important regulators of vascular pathogenesis. Conversely, OPG production is inhibited by peroxisome proliferator-activated receptor-γ ligands in VSMCs,32 agents associated with anti-inflammatory and antiatherogenic effects in vitro and in vivo. Thus, the picture emerging from these previous studies is quite complex: although a basal constitutive production/release of OPG by vascular smooth muscle cells and endothelial cells is essential to protect the vessel wall against calcification, an increased release of OPG correlates to increased cardiovascular risk.11-18
In this study, we have demonstrated for the first time that the treatment of vascular endothelial cells with recombinant OPG promotes the adhesion of both primary PMNs and leukemic HL60 cells to the endothelial cell surface. It is noteworthy that the greatest effect was observed at concentrations of OPG in the same range of those described to be elevated in the serum of patients affected by cardiovascular diseases.11-18 The ability of OPG to promote leukocyte adhesion to endothelial cells was very rapid (5-15 minutes). A similar increase of pro-adhesion activity was observed when PMNs rather than HUVECs were pretreated with OPG before the coculture incubation. In this respect, it is noteworthy that both PMNs and HL60 cells exhibit significant surface levels of both RANKL and TRAIL, whereas endothelial cells, obtained by different sources, did not show surface expression of the high-affinity transmembrane ligands of OPG. Therefore, OPG is likely to interact through its ligand-binding domain with RANKL and/or TRAIL expressed on the surface of leukocytes. On the other hand, the data obtained pretreating OPG with heparin before the adhesion assays strongly suggest that OPG interacts with endothelial cell membrane through its heparin-binding domain. In this respect, it should be mentioned that the heparan sulfates expressed on the cell surface are important participants in cell-surface signaling and have been involved in actin cytoskeleton regulation, cell adhesion and migration, and modulation of specific receptor interactions.37,38 It should also be underlined that the interaction between OPG and glycosaminoglycans was confirmed in a recent study by surface plasmon resonance, which demonstrated that OPG binds to heparin with a high affinity (Kd, 0.28 nM).28
Using intravital microscopy, which provides a useful tool for observing leukocyte-endothelium interactions in vivo, we have also demonstrated that the findings obtained in vitro are relevant in vivo because leukocytes circulating in the rat mesentery under physiological flow conditions firmly adhered to the endothelium of the postcapillary venules after the topical administration of recombinant OPG. Because an abnormal increase of leukocyte adhesion to endothelial cells is considered an early step in endothelial cell dysfunction,39 the results illustrated in our study suggest a potential mechanism to explain why pathologically elevated serum OPG levels are linked to the development or status of vascular disease. In fact, a number of reports have shown that increased circulating OPG levels often occur in cardiovascular diseases.11-18 Moreover, linkage of 2 OPG genetic polymorphisms was associated with an increased risk of coronary artery disease in white men, and serum OPG levels correlated with one of these polymorphisms.40
Studies of other authors have shown that OPG is an NF-κB-inducible gene, whose expression and release in culture of vascular cells is significantly increased by inflammatory cytokines.30,33 In this respect, we have confirmed that TNF-α potently induced the release in HUVEC culture of endogenous OPG. Although it cannot be excluded that the increased release of OPG in response to inflammatory cytokines might merely represent a bystander effect or even an attempt to counteract the endothelial damage induced by inflammatory cytokines, our present data suggest rather that elevated levels of OPG might substantially contribute to establish a vicious inflammatory circle by enhancing the proadhesive activity of TNF-α. Because we have recently demonstrated that the systemic administration or recombinant TRAIL shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice,41 an alternative but not mutually exclusive mechanism by which elevated levels of serum OPG might contribute to cardiovascular risk is by inhibiting the antiatherosclerotic activity of circulating TRAIL.
Our present findings are particularly noteworthy also considering the enormous surface area of the endothelium throughout the body, which suggests that endothelial cells are a key cell type involved in the production/release of circulating OPG in human serum.33,42 Although previous studies have reported the possibility that OPG may affect relevant cell parameters, such as release of matrix metalloproteinase-9 (MMP-9) activity,34 this is the first study, to the best of our knowledge, that demonstrates the ability of OPG to markedly increase the leukocyte-endothelial interactions both in vitro and in vivo. Overall, our study suggests how the enhanced OPG production and release associated to pathologic conditions may contribute to the inflammatory status of endothelium characterizing cardiovascular disease and tumor angiogenesis. Therefore, a suggestion deriving from our study is that therapeutic strategies aimed to decrease the OPG serum levels may be suitable for improving the vascular function in vascular pathologic conditions characterized by a chronic inflammation state.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (AIRC), the Italian Ministry of University and Research (Programmi di Ricerca di Interesse Nazionale and Fondo per gli Investimenti della Ricerca di Base RBIN045LT8) and the CrTrieste Foundation.
Authorship
Contribution: G.Z., P.S., and F.T. designed the study, analyzed the data, and wrote the manuscript; F.C., F.B., P.D., F.F., and C.C. performed research and contributed analytical tools.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Giorgio Zauli, MD, PhD, Department of Biomedicine, University of Trieste, Via Manzoni 16, 34138 Trieste, Italy; e-mail: zauli@units.it.