Abstract
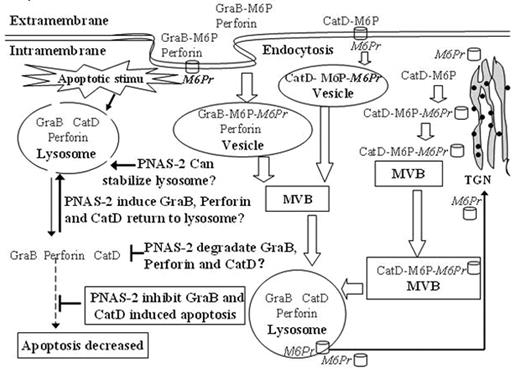
We used functional analyses of PNAS-2 by RNAi and RNA overexpression and demonstrated PNAS-2 as an anti-apoptotic gene. PNAS-2 expression was significantly increased in de novo and relapsed acute leukemia patients, but in patients in complete remission, the expression of PNAS-2 decreased to the levels comparable to those found in normal controls. In carcinomas, PNAS-2 expression was not up-regulated, indicating that PNAS-2 overexpression was specific for leukemia. So we hypothesized that PNAS-2 could act as an oncogene and anti-apoptotic gene which probably participates leukemogenesis (Hai-Rong WANG, et al. Oncology, Accepted). To study the subcellular localization of PNAS protein, we constructed PNAS-2-GFP expression plasmids and transfected them into U937 leukemic cell line. We found that PNAS-2-GFP fusion protein not only located in vesicles near the nucleus but also distributed throughout the cytosol instead of Diane’s report that CHMP5/PNAS-2 was found in vesicles near the nucleus in Cos-7 cells. This phenomenon indicates the abnormal location of PNAS-2 protein either in U937 leukemia cells or in Cos-7 cells. For the protein classification and secondary structure prediction system: SOSUI showed that PNAS-2 wasn’t a membranal protein and the function of CHMP5/PNAS-2 protein was involved in the lysosome and MVB (multivesicular body), so PNAS-2 should be a protein in the interior of the MVB and lysosome, thus abnormal location of PNAS-2 protein might occur in U937 leukemic cells. From our data and related articles, PNAS-2 served as an anti-apoptotic gene and located in MVB and lysosome. We suggested that PNAS-2 might execute its anti-apoptotic function via following ways: it might be a protease which could degrade apoptogenic factors such as CatD, GraB and perforin in MVB and lysosome; or it could return CatD, GraB and perforin to the lysosome; or it could stabilize the membrane of MVB and lysosome. When PNAS-2 was set free into the cytosol from MVB structure in leukemic cells, it would enhance its anti-apoptotic function via enhanced above-mentioned ways.
Now compelling evidences showed that insufficient apoptosis could result in tumor genesis, thus we thought abnormal subcellular location of PNAS-2 in leukemic cells might participate in leukemogenesis. Antibody microarray was used to study the protein changes after inhibition of PNAS-2, we found cancer suppressor proteins such as p53, Rb increased after PNAS-2 was suppressed, while the rest cancer suppressor proteins did not change, indicating the overexpression of PNAS-2 might participate in tumorigeness by suppressing tumor suppressor proteins. Of notice, Granzyme B-perforin apoptotic pathway was inhibited when PNAS-2 was up-regulated. Granzyme-mediated apoptosis was part of the immune surveillance mechanism where malignantly transformed cells were targeted by CTL and NK cells before cancer could ensue. In conclusion, the abnormal location of PNAS-2 in leukemic cells could enhance its anti-apoptotic function. PNAS-2 might participate in leukemogenesis via its inhibition of apoptosis, cancer suppressor proteins and Granzyme B-perforin pathway.
Author notes
Disclosure:Research Funding: 1, Research Fund from Chinese National Natural Science Foundation. (NO. 30670881). 2, Research Fund from Shanghai Municipal Education Commission. (NO. 05BZ37). Off Label Use: 1, Research Fund from Chinese National Natural Science Foundation. (NO. 30670881) 2, Research Fund from Shanghai Municipal Education Commission. (NO. 05BZ37).