Abstract
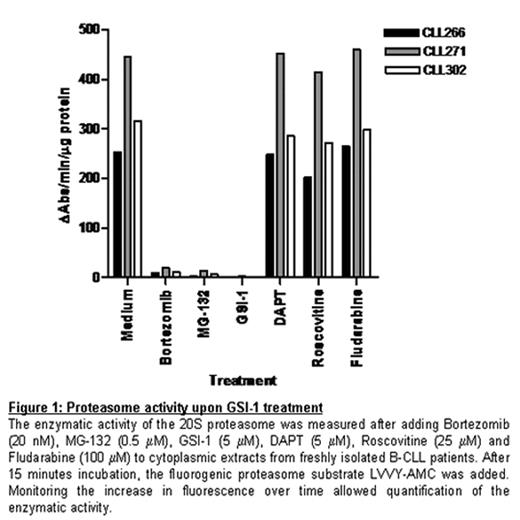
Mainly based on the observation that overexpression of CD23 in B-CLL cells is regulated by Notch2, deregulation of the Notch pathway has been suggested to contribute to the pathogenesis of B cell chronic lymphocytic leukemia (B-CLL). The aim of the present study was to assess a possible functional role of the Notch pathway in CLL. To this end we performed two kind of experiments. First we co-cultured primary CLL cells with either L cells or OP9 cells expressing the Notch ligands DeltaLike1 (DL1) and Jagged1 (Jag1). This did not affect cell survival in CLL. Next we evaluated the effect of the g-secretase inhibitors GSI-1 and GSI-9 (DAPT) which have been shown to block intracellular processing of all four Notch receptors. Here we encountered a surprising dichotomy: whereas DAPT was unable to induce apoptosis in CLL, it did inhibit lineage commitment of early thymic precursors by means of DL-1 triggering of the Notch1 receptor (Dontje et al, Blood 15 March 2006, Vol. 107, No. 6). In contrast we found that GSI-1 was a potent inducer of apoptosis in CLL, but did not affect Notch1 dependent lineage commitment, indicating that GSI-1-induced apoptosis in B-CLL is not due to inhibition of Notch signaling. Instead, we observed efficient GSI-1 mediated blocking of the proteasome, measured using a 20S proteasomal activity assay (Fig. 1). The blocking activity was equivalent to that observed with two well known proteasome inhibitors, Bortezomib and MG-132. In contrast DAPT had no effect on proteasome activity. Furthermore, GSI-1-induced apoptosis was associated with a transcription-independent accumulation of the BH3-only protein Noxa. The pivotal role of Noxa in GSI-1 mediated apoptosis was demonstrated via RNAi in a model system. Importantly, p53 functionality proved not to be required for GSI-1-induced apoptosis. In summary, we have shown that GSI-1 efficiently blocks the proteasome and that this induces p53-independent apoptosis in B-CLL. Therefore, GSI-1, or similar compounds that inhibit g-secretase activity, may be an effective treatment option in B-CLL.
Proteasome activity upon GSI-1 treatment The enzymatic activity of the 20S proteasome was measured after adding Bortezomib (20 nM), MG-132 (0.5 μM), GSI-1 (5 μM), DAPT (5 μM), Roscovitine (25 μM) and Fludarabine (100 μM) to cytoplasmic extracts from freshly isolated B-CLL patients. After 15 minutes incubation, the fluorogenic proteasome substrate LVVY-AMC was added. Monitoring the increase in fluorescence over time allowed quantification of the enzymatic activity.
Proteasome activity upon GSI-1 treatment The enzymatic activity of the 20S proteasome was measured after adding Bortezomib (20 nM), MG-132 (0.5 μM), GSI-1 (5 μM), DAPT (5 μM), Roscovitine (25 μM) and Fludarabine (100 μM) to cytoplasmic extracts from freshly isolated B-CLL patients. After 15 minutes incubation, the fluorogenic proteasome substrate LVVY-AMC was added. Monitoring the increase in fluorescence over time allowed quantification of the enzymatic activity.
Author notes
Disclosure: Research Funding: KWF.