Abstract
Vitamin K is a cofactor in the production of blood coagulation factors (in the liver), osteocalcin (in bone), and matrix Gla protein (cartilage and vessel wall). Accumulating evidence suggests that for optimal bone and vascular health, relatively high intakes of vitamin K are required. The synthetic short-chain vitamin K1 is commonly used in food supplements, but recently the natural long-chain menaquinone-7 (MK-7) has also become available as an over-the-counter (OTC) supplement. The purpose of this paper was to compare in healthy volunteers the absorption and efficacy of K1 and MK-7. Serum vitamin K species were used as a marker for absorption and osteocalcin carboxylation as a marker for activity. Both K1 and MK-7 were absorbed well, with peak serum concentrations at 4 hours after intake. A major difference between the 2 vitamin K species is the very long half-life time of MK-7, resulting in much more stable serum levels, and accumulation of MK-7 to higher levels (7- to 8-fold) during prolonged intake. MK-7 induced more complete carboxylation of osteocalcin, and hematologists should be aware that preparations supplying 50 μg/d or more of MK-7 may interfere with oral anticoagulant treatment in a clinically relevant way.
Introduction
Vitamin K is a group name for a number of structurally related compounds including phylloquinone (vitamin K1) and menaquinones (K2 vitamins). Menaquinones are classified according to the length of their aliphatic side chain and are designated as MK-n, where n stands for the number of isoprenoid residues in that chain. The function of all forms of vitamin K is that they serve as a cofactor for the posttranslational carboxylation of certain protein-bound glutamate residues, which are converted into gamma-carboxy glutamate (Gla). These Gla residues form calcium-binding sites that are essential for the activity of the proteins in which they are found.1 During gamma-glutamate carboxylation, vitamin K is oxidized into its epoxide form (KO), which is reconverted to vitamin K quinone (K) by the enzyme vitamin K epoxide reductase (VKOR). Derivatives of 4-hydroxycoumarin (including warfarin and acenocoumarol) specifically inhibit VKOR, thus preventing the recycling of vitamin K.2
Well-known Gla-containing proteins are the blood coagulation factors II, VII, IX, and X, which are all synthesized in the liver. Gla proteins not related with blood clotting are osteocalcin (synthesized in bone) and matrix Gla protein (primarily synthesized in cartilage and in the vessel wall).3 Although in some cases conflicting data were obtained, accumulating evidence suggests that low vitamin K intake is associated with low bone mineral density,4,5 increased fracture risk,6,7 and increased risk of cardiovascular disease and mortality.8 Moreover, vitamin K supplements were shown to slow down the rate of postmenopausal bone loss9 and to block age-related arterial stiffening.10 Therefore, increasing the dietary vitamin K intake is potentially beneficial for bone and vascular health.11
In food, the most important K vitamins are K1 (notably found in green vegetables and some plant oils) and the long-chain menaquinones MK-7, MK-8, and MK-9 (present in fermented foods, notably cheese and natto).12-14 Natto consists of fermented soy beans and is a popular food in Japan, but because of its strong taste it is not well appreciated elsewhere. It is the richest dietary source of vitamin K presently known, almost all of which occurs in the form of MK-7. In food supplements, 3 forms of vitamin K may be found: MK-4 (prepared by organic synthesis and almost exclusively used in Japan), K1 (also synthetic and the predominant form used in the rest of the world), and MK-7 (a natural form prepared by extraction of natto food). Since the molecular structures of K1 and MK-4 are comparable (both contain 4 isoprenoid residues, 3 of which are saturated in K1 but contain a double bond in MK-4), their physico-chemical characteristics are closely similar. The higher menaquinones including MK-7 are much more hydrophobic, however, and in vivo they are handled differently: they have longer half-life times,13 and in the circulation they are incorporated into low-density lipoproteins.15 K1 is by far the most common form of vitamin K in commercially available supplements, but because of the health claims for the regular consumption of natto that are repeatedly made in the scientific literature,16-18 MK-7 in the form of a natto extract is rapidly gaining interest. Whereas supplements containing vitamin K1 have been commercially available for many years, MK-7 has entered the market only recently. Hence comparative data on pharmacokinetics, coenzyme activity, and potential interference with vitamin K antagonists (oral anticoagulants) for supplemental vitamin K1 and MK-7 are presently not available.
The aims of this paper were (1) to compare the absorption and efficacy of K1 and MK-7 in order to provide a rational basis for the choice between functional foods and food supplements containing either K1 or MK-7; and (2) to increase the awareness of hematologists for the potential interference of this new line of over-the-counter (OTC) products with the management of patients on oral anticoagulant treatment.
Materials and methods
Volunteers
All volunteers (equal numbers of men and women) were apparently healthy and between 25 and 35 years old. For single-dose experiments, vitamin K was given as an oil solution that formed part of the breakfast and which was consumed at the institute. For long-term experiments, vitamin K1 tablets (prepared at a local pharmacy) and MK-7 capsules were handed out to the participants to be consumed once daily together with either breakfast or dinner (as indicated). MK-7 both in the form of an oil solution and in capsules was from J-Oil Mills (Sumitomo, Tokyo, Japan). In all experiments the volunteers were requested not to consume vitamin K–rich foods (spinach, kale, Brussels sprouts, and broccoli) starting at 2 days before and during the experiment. Blood (10 mL) was taken by venipuncture to prepare either serum or citrated plasma (as indicated). In absorption studies, the vitamin K concentrations are expressed per unit of weight; to compensate for their different molecular weights, the vitamin K doses and concentrations in functional studies are given on a molar base. Approval for these studies was obtained from the University of Maastricht and University Hospital Medical Ethics Committee review board. Informed consent was provided in accordance with the Declaration of Helsinki.
Study designs
Study 1: bio-availability after oral intake.
MK-7 (1500 mg/L) in the form of an oil solution was a kind gift from N-ZymeCeuticals (Longmont, CO). K1 (Konakion; Roche, Basel, Switzerland) was dissolved in the oil, which was supplemented with corn oil to give a mixture containing 100 μg/mL each of the 2 vitamins. Ten milliliters of this solution was added to 20 mL of orange juice, emulsified by vigorous shaking, and consumed during a standard breakfast containing 30 g of fat. Fifteen volunteers were enrolled in this study. Blood was taken at baseline and at t = 0, 2, 4, 6, 8, 24, 48, 72, and 96 hours after mealtime to prepare serum. The area under the curve (AUC) of serum vitamin K levels was taken as a measure for absorption and bio-availability. The AUC was calculated from the following equation: AUC = Σ(xn − xn−1)(yn + yn−1)/2, where xn and xn−1 are 2 adjacent values on the horizontal axis, and yn and yn−1 are the corresponding values on the vertical axis.
Study 2: dose-response relation.
MK-7 oil and K1 were mixed to a final concentration of 100 mg/L and this mixture was given at increasing dosages (50, 100, 150, 200, 250, 300, and 500 μg each of the 2 vitamins) together with a standard breakfast. Ten volunteers were enrolled for this experiment. Blood was taken at baseline and at 4 and 24 hours after mealtime to prepare serum. Each single dose was followed by a 2-week wash-out period before increasingly higher doses were administered.
Study 3: osteocalcin carboxylation during prolonged intake.
Eighteen volunteers were randomized to take 0.22 μmol/d of either K1 or MK-7 in a crossover design. The treatment with each vitamin was 6 weeks with a wash-out period of 12 weeks. Blood sampling at 8 am was done at baseline and at days 3, 7, 14, 21, 28, 35, and 42; the supplements were taken during dinner between 6 and 7 pm. K vitamins and the bone Gla protein osteocalcin were monitored in serum. Osteocalcin (OC) carboxylation was used as an efficacy marker for vitamin K, and the ratio between carboxylated osteocalcin (cOC) and uncarboxylated osteocalcin (ucOC) was calculated to monitor vitamin K status. At baseline, the cOC/ucOC ratio was 1.74 (mean value).
Study 4: interference with oral anticoagulants.
Twelve subjects (mean age: 34.2 ± 6.4 years; mean body mass index: 23.5 ± 2.6) were treated with acenocoumarol and reached the target international normalized ratio (INR) value of 2.0 within 3 weeks. Different doses (range, 1.8-4.5 mg) were required for each participant to reach the same level of anticoagulation. After stabilization, each subject received the same individualized acenocoumarol dose throughout the study. While the anticoagulant treatment was continued, the subjects received a constant daily supplemental dose of vitamin K during the first week and increased doses during subsequent weeks. At the end of each week, blood was taken for analysis. For K1, the starting dose was 110 nmol/d with a weekly increment of 110 nmol/d; after the last dose there was a 2-week wash-out period and it was checked that the INR values had returned to their target value of 2.0. Subsequently, the participants received MK-7 with a starting dose of 150 nmol and a weekly increment of 150 nmol/d.
Laboratory tests
K vitamins were measured in serum as described previously.13 In short, samples were extracted with hexane and after a prepurification on silica columns they were analyzed by high-performance liquid chromatography (HPLC) using a reversed-phase column with online zinc reduction and fluorescence detection. Vitamin K1-25 (a synthetic form of vitamin K1 containing 5 isoprenoid residues) was used as internal standard. cOC and ucOC were quantified in serum with the Gla-OC and Glu-OC test kits from Takara (Shiga, Japan). The INR value was measured at the Department of Hematology of the University Hospital Maastricht and is generally regarded as the gold standard for monitoring anticoagulation. Nontreated subjects have an INR value of 1.0, and during anticoagulation this value increases in a dose-dependent way. The procoagulant activity of prothrombin and factor VII (FIIc and FVIIc, respectively) were measured in a coagulometer (ACL 300 Research; Instrumentation Laboratory, Milan, Italy) using Thromborel S and human coagulation factor II– and VII–deficient plasma (Behringwerke AG, Marburg, Germany). All values were expressed as a percentage of the value obtained for pooled normal plasma from 60 healthy volunteers.
Data handling
Mean values of the study group are given for each experiment; error bars represent standard deviation. The 2-sided Mann-Whitney test was performed to determine the significance of differences between datasets obtained for both vitamins at indicated time points; mean group values were considered to be different at P values less than .05.
Results
Study 1
A mixture of vitamins K1 and MK-7 (1 mg of each) in corn oil was ingested by 15 volunteers and blood was taken at regular intervals. In Figure 1, the circulating K vitamins are plotted as a function of time. For both forms of vitamin K, the peak values were seen at approximately 4 hours after mealtime, followed by a rapid decline, which probably represents redistribution and tissue uptake. At 8 hours after mealtime, vitamin K1 had returned to near-baseline levels, whereas MK-7 was still present in considerable amounts. The decline of MK-7 was biphasic and slow between 8 and 96 hours. Although the available data points do not allow accurate half-life time assessment, the decline of K1 was 86% in 4 hours, which is consistent with the reported short half-life time (1-2 h) for K1.13,19,20 An estimate of the half-life time for MK-7 was obtained from the slope of the second phase of the decline (50% loss in 68 h), which is consistent with its reported very long half-life time (about 3 days).13 In studies 2 to 4, we have investigated whether differences can also be found between the 2 K vitamins with respect to other characteristics, including circulating vitamin K concentrations at daily intake and efficacy of protein carboxylation.
Circulating vitamin K concentrations following a single oral dose of 1 mg each of vitamin K1 and MK-7. Points are means from 15 subjects; error bars represent SD. Baseline levels (1 nM [0.45 μg/L] for K1 and < 0.07 nM [< 0.05 μg/L] for MK-7) were subtracted from all values. ▪ indicates K1; and ○, MK-7.
Circulating vitamin K concentrations following a single oral dose of 1 mg each of vitamin K1 and MK-7. Points are means from 15 subjects; error bars represent SD. Baseline levels (1 nM [0.45 μg/L] for K1 and < 0.07 nM [< 0.05 μg/L] for MK-7) were subtracted from all values. ▪ indicates K1; and ○, MK-7.
Study 2
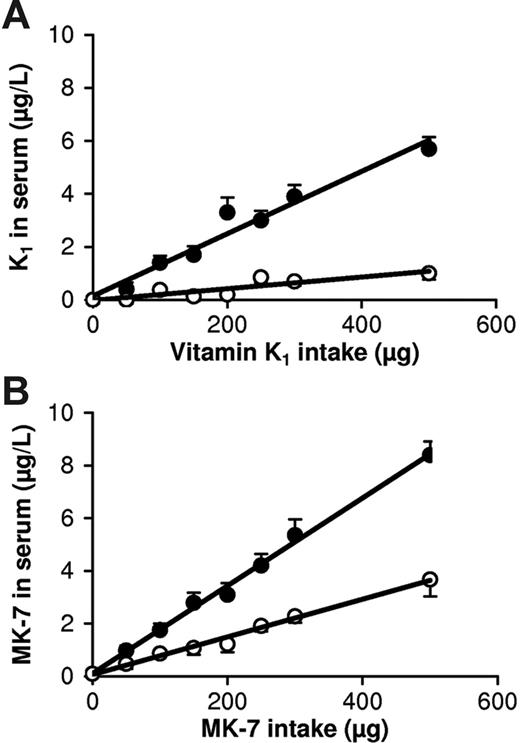
Ten volunteers received increasing doses of K1 and MK-7 as part of their breakfast. At 4 hours after mealtime, both vitamins showed a linear dose response, with a slightly steeper curve for MK-7 (Figure 2). At 24 hours after mealtime, however, there was no response for K1 up to a dose of 200 μg, whereas at 100 μg the circulating MK-7 concentration was approximately 1.5 nM (1 μg/L), which is the upper limit of the normal range for total serum vitamin K (sum of K1 + K2).
Dose-response curves for K1 and MK-7 at 4 and 24 hours following a single oral dose. (A) Curves for K1; (B) curves for MK-7. Points are means from 10 volunteers; error bars represent SD. The difference between K1 and MK-7 at 4 hours was statistically significant at doses of at least 150 μg; at 24 hours the difference was significant at all doses. • indicates serum vitamin K concentrations at 4 hours; and ○, serum vitamin K concentrations at 24 hours after mealtime.
Dose-response curves for K1 and MK-7 at 4 and 24 hours following a single oral dose. (A) Curves for K1; (B) curves for MK-7. Points are means from 10 volunteers; error bars represent SD. The difference between K1 and MK-7 at 4 hours was statistically significant at doses of at least 150 μg; at 24 hours the difference was significant at all doses. • indicates serum vitamin K concentrations at 4 hours; and ○, serum vitamin K concentrations at 24 hours after mealtime.
Study 3
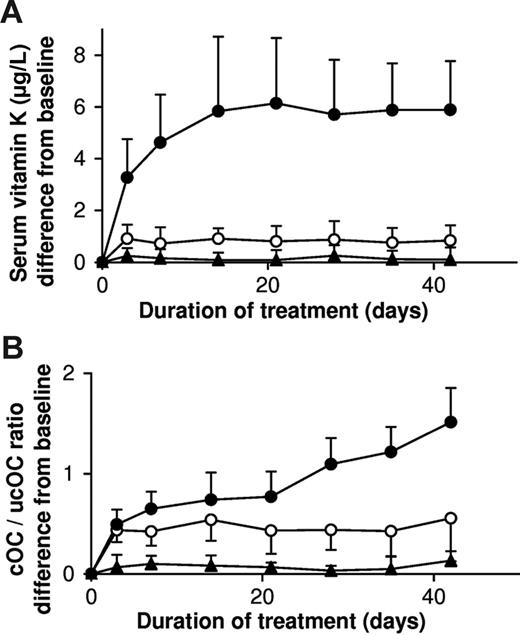
For this experiment we used tablets containing 0.22 μmol of vitamin K1 and capsules with a natto extract containing 0.22 μmol of MK-7. It was verified that the absorption of both vitamins from these products was comparable to the absorption from corn oil (data not shown). As is demonstrated in Figure 3A, MK-7 accumulated during the first 2 weeks until it reached a plateau level of about 10 nM (6 μg/L), whereas K1 remained slightly above the placebo values during the entire study period. The efficacy of both K vitamins for osteocalcin carboxylation was monitored using the ratio between circulating carboxylated osteocalcin and uncarboxylated osteocalcin (cOC/ucOC), and it turned out that within 3 days both vitamins had induced increased osteocalcin carboxylation but that only by taking MK-7 did the effect continue to increase during the entire study period (Figure 3B).
Accumulation and efficacy of K vitamins during long-term daily administration. Participants received in a crossover design either K1 (○) or MK-7 (•) or placebo; in the latter case only K1 (▴) could be detected. Points are means of 18 values; error bars represent SD. (A) Circulating levels of vitamin K; baseline levels for K1 were subtracted; no MK-7 could be detected at baseline. (B) Ratio between circulating carboxylated and undercarboxylated osteocalcin (cOC/ucOC); at baseline the ratio was 1.74 for MK-7, 1.8 for K1, and 1.7 for the placebo group.
Accumulation and efficacy of K vitamins during long-term daily administration. Participants received in a crossover design either K1 (○) or MK-7 (•) or placebo; in the latter case only K1 (▴) could be detected. Points are means of 18 values; error bars represent SD. (A) Circulating levels of vitamin K; baseline levels for K1 were subtracted; no MK-7 could be detected at baseline. (B) Ratio between circulating carboxylated and undercarboxylated osteocalcin (cOC/ucOC); at baseline the ratio was 1.74 for MK-7, 1.8 for K1, and 1.7 for the placebo group.
Study 4
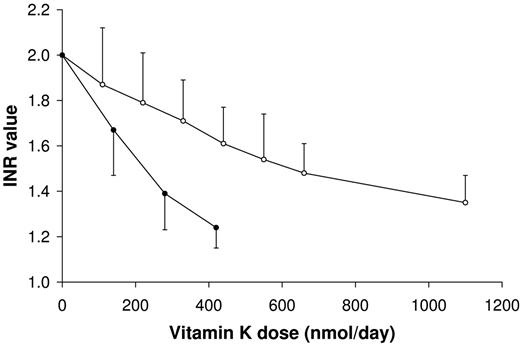
Coumarin derivatives act as vitamin K antagonists and are often used for oral anticoagulant treatment. In the study shown in Figure 4, we have monitored to what extent intake of K1 and MK-7 may interfere with this medication. From the data obtained it was calculated that only at a dose of 700 nmol/d (315 μg/d) of K1 had the INR value decreased from 2.0 to 1.5. The plasma prothrombin and coagulation factor VII procoagulant activities during the entire period of oral anticoagulant treatment and vitamin K supplementation are given in Table 1. MK-7 turned out to be much more potent, and a comparable decrease in INR was reached at an intake of 200 nmol/d (130 μg/d).
Interference of K vitamins with oral anticoagulants. Participants were treated with acenocoumarol until they reached a stable target INR level of 2.0. Subsequently they received a daily dose of vitamin K (as indicated) for 1 week. At the end of the week blood was taken by venipuncture, and the vitamin K dose was increased during the next week. Points are means of 12 values; error bars represent SD. ○ indicates K1; and •, MK-7.
Interference of K vitamins with oral anticoagulants. Participants were treated with acenocoumarol until they reached a stable target INR level of 2.0. Subsequently they received a daily dose of vitamin K (as indicated) for 1 week. At the end of the week blood was taken by venipuncture, and the vitamin K dose was increased during the next week. Points are means of 12 values; error bars represent SD. ○ indicates K1; and •, MK-7.
Discussion
In this paper we have compared the in vivo properties of 2 forms of vitamin K: MK-7 and K1. We demonstrate that after oral ingestion, MK-7 is more effective in both catalyzing osteocalcin carboxylation in bone and counteracting coumarin anticoagulants in the liver. The mechanism underlying this observation may be MK-7's much longer half-life time in the circulation and its reported 6-fold higher cofactor activity in vitro.21
Despite their different lipophylicity, maximal serum concentrations of K1 and MK-7 were seen at approximately 4 hours after intake, followed by a steep decline. A marked difference was observed during the second phase (between 8 and 96 h after mealtime), in which K1 declined to its baseline level within several hours, whereas MK-7 remained detectable for at least 4 days (which was the last point of measurement). The difference between the 2 vitamins may be related to the fact that following intestinal absorption, both are taken up in the triglyceride fraction from which they are rapidly cleared by the liver but that only higher menaquinones are redistributed via low-density lipoproteins.15 A consequence of the long half-life time of MK-7 is that it is available longer than K1 for uptake by extrahepatic tissues. If expressed as AUC over 24 hours (AUC24), the availability of MK-7 is 2.5-fold better than that of K1; if expressed as AUC96, it is even 6-fold better.
For both K vitamins linear dose-response curves were obtained for intakes between 0 and 500 μg. If measured at 4 hours after intake, the response of MK-7 was only slightly (but significantly) higher than that of K1. At 24 hours, however, no effect of K1 could be observed after a 200-μg dose (ie, twice the recommended daily allowance), whereas at 100 μg of MK-7 the circulating vitamin K concentration was 1.5 nM (1 μg/L), which is the upper limit of the normal range for total serum vitamin K. This means that if taken in single daily doses of 100 μg, only MK-7 is effectively present in the circulation and available for absorption by various tissues during the 24 hours following intake. This is consistent with the data in Figure 3A, showing that if taken on a regular basis, there is no accumulation of K1, whereas MK-7 accumulates asymptotically during the first 2 weeks, after which a steady-state level is reached. At comparable intakes, the final level of MK-7 was 7- to 8-fold higher than that of K1, suggesting that if taken on a daily basis, 25 μg/d of MK-7 is more efficacious than 100 μg/d of K1.
The extent of osteocalcin carboxylation is generally thought to be the most sensitive marker for human vitamin K status and is often expressed as the ratio between carboxylated and undercarboxylated osteocalcin. In the healthy adult population, about 30% of the circulating osteocalcin occurs in its undercarboxylated form,5,22 and increased vitamin K intake results in a rapid decline of ucOC,9,23 suggesting a state of subclinical vitamin K deficiency in healthy bone tissue. In a study designed to compare the efficacy of equimolar amounts of K1 and MK-7, we measured the degree of osteocalcin carboxylation after a 6-week period of vitamin K intake. A small increase of osteocalcin carboxylation was visible for both forms of vitamin K at day 3, but whereas the effect of K1 remained constant after that time, that of MK-7 inclined during the entire 6 weeks of treatment. At the end of the study, the change in the cOC/ucOC ratio was 3 times higher for MK-7 than for K1, suggesting that the higher serum levels of MK-7 reflect higher tissue levels and better utilization of MK-7.
The vitamin K–dependent clotting factors are all produced in the liver and, in contrast to osteocalcin, they are all fully carboxylated in the healthy population. For efficacy comparison of K vitamins in the liver, we therefore used an artificial model of hepatic vitamin K deficiency (ie, mildly anticoagulated volunteers). In this study population, we monitored to which extent the 2 forms of vitamin K were capable of counteracting the effect of coumarin anticoagulants. It turned out that if expressed on a molar basis, MK-7 is a 3 to 4 times more potent antidote for oral anticoagulation than is K1. If expressed per weight, the efficacy of MK-7 in the liver is still 2.5 times higher than that of K1. In a previous paper we demonstrated that vitamin K1 supplements containing no more than 100 μg/d are not likely to result in clinically relevant disturbances of oral anticoagulant therapy.22 Extrapolating these figures, it may be concluded that MK-7 supplements containing more than 50 μg/d may interfere with oral anticoagulant treatment, whereas doses of at least 50 μg are not likely to affect the INR value in a relevant way. Since our study did not include intakes below 100 μg/d, more elaborate research including a larger number of volunteers and dosages between 0 and 100 μg/d are required to confirm this conclusion.
Since oral anticoagulants block the recycling of vitamin K, the extent of utilization can be monitored via measuring vitamin K epoxides, which are end products of the carboxylation reaction. Based on the assumption that the clearance of the various epoxides is comparable, their serum levels will reflect the extent to which the various K vitamins have been used. Since MK-7 was demonstrated to have a much longer half-life time than K1, it cannot be excluded that MK-7 epoxide also has a longer half-life time than K1 epoxide. To correct for this potential bias, we have used the ratio between the epoxide and the quinone form of the 2 K vitamins. It resulted that at a wide range of intakes, the KO/K ratio was 3-fold higher for MK-7 than for K1. This strongly suggests that MK-7 is utilized better than K1.
Taken together, these data demonstrate considerable differences between MK-7 and K1: higher and more stable serum levels are reached with MK-7, and MK-7 has a higher efficacy in both hepatic and extrahepatic protein carboxylation. During recent years many studies have demonstrated that the extrahepatic vitamin K requirement exceeds the recommended daily allowance (100-120 μg/d) for vitamin K1. For the food industry, an alternative to increasing the recommended dose would be introducing on a larger scale the more potent MK-7 instead of K1 in functional foods and multivitamin supplements. Hematologists, on the other hand, need to be aware that relatively low doses of MK-7 may have a larger impact on the stability of oral anticoagulation than vitamin K1. Obviously, a large study in patients on oral anticoagulant treatment is needed to demonstrate the safety of even low doses of MK-7 in this population. Until that time, we propose to use an upper safety limit for intake of 50 μg/d for long-chain menaquinones (including MK-7) in patients on oral anticoagulant treatment. This dose is comparable with the menaquinone content of 75 to 100 g of cheese13 ; by intrapolation of the curve in Figure 4, such amount would lead to a disturbance of the INR value of no more than 10%, which may be regarded as tolerable in the management of oral anticoagulant therapy. On the other hand, its long half-life time suggests that regular intake of MK-7 in combination with properly adapted coumarin doses may result in more stable INR values.
Authorship
Contributions: K.J.F.T. and M.H.J.K. performed the research; K.H. acted as medical supervisor and is responsible for the clinical interpretation of data; H.V. and C.V. designed the research; and L.J.S. and C.V. supervised the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: By the time this study was performed, H.V. was the scientific advisor of Natural ASA, the company that contributed financially to this study. The design originated from the concern that MK-7, a new form of vitamin K, might have an unexpected high potency and interfere with oral anticoagulant therapy. Natural ASA has stopped the marketing of the product tested, however. Presently, H.V. is a board member of NattoPharma, a company still involved in the marketing of the MK-7 containing natto extract. The remaining authors declare no competing financial interests.
Correspondence: C. Vermeer, Dept of Biochemistry, University of Maastricht, PO Box 616, 6200 MD Maastricht, The Netherlands; e-mail: c.vermeer@bioch.unimaas.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by research funding from Natural ASA, Lysaker, Norway (C.V.).

![Figure 1. Circulating vitamin K concentrations following a single oral dose of 1 mg each of vitamin K1 and MK-7. Points are means from 15 subjects; error bars represent SD. Baseline levels (1 nM [0.45 μg/L] for K1 and < 0.07 nM [< 0.05 μg/L] for MK-7) were subtracted from all values. ▪ indicates K1; and ○, MK-7.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-08-040709/4/m_zh80080711200001.jpeg?Expires=1768153113&Signature=PjixkWKHEy5jLMsuTwcM7engXOGFS0iZ0z15QPi9VKMe-DufG9cijLp1zCePYgqVAYMp6qYtrPX~FwtOU6X2MI8gGjH-uLFsBkzs1Z~wIwQb5knxyl92OJ25JxJzMySvJxFsgh2Og95EQ4cDkRGvy4hKOyq-BEIOECp3G2vRCg6nd68YOq8llahI9iWa7bm-ttjpFdcmz~d7-iG1lH9mVMsRPWgOBBYOaZ5~dRWAi4rF3Nw-1O~Ao4encHlxF75tZZpjQR7NeYJ4-SEg0Dv-W28tIj-E9IKKQmM1UA26UBXhBNsbXYohkd~bGvcT-VQ1FfEdgVSsq4xp5Q7ZUq4acQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)