The advent of imatinib mesylate has revolutionized the treatment of chronic myeloid leukemia (CML). However, at 5 years, 15% to 20% of patients develop some form of resistance and another 5% discontinue because of toxicity.
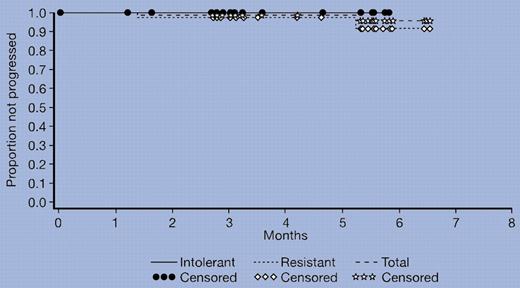
In this issue, Hochhaus and colleagues have reported on the remarkable ability of dasatinib to induce major hematologic and cytogenetic responses in chronic myeloid leukemia (CML) patients who have failed prior therapy with imatinib. Dasatinib induced complete hematologic remission in 90% of patients and major cytogenetic responses in 52% of patients. The cytogenetic responses were maintained in 96% of patients with imatinib-resistant disease and in 100% of patients who were intolerant to imatinib (see figure). Based on experience with CML, one may presume that these results will affect overall survival. These data are indeed impressive, justifying the early publication of the initial cohort of 186 of patients, although the median follow-up was only 8 months and more than twice this number of patients enrolled on this study.
Duration of major cytogenetic response associated with dasatinib treatment. See the complete figure in the article beginning on page 2303.
Duration of major cytogenetic response associated with dasatinib treatment. See the complete figure in the article beginning on page 2303.
The development of imatinib for the treatment of CML represents the most important advance of any new therapy for leukemia in the past decade and serves as the paradigm for targeted therapies. Almost before the ink was dry on this milestone, it became clear that imatinib, while altering the standard of care for most patients, will be of limited benefit to patients who develop imatinib-resistant mutations. Furthermore, patients who are intolerant to imatinib have limited therapeutic options. Dasatinib is a new oral small-molecule tyrosine kinase inhibitor that has been granted accelerated approval for use in adult patients with CML or Philadelphia chromosome–positive acute lymphoblastic leukemia (ALL) who are resistant or intolerant to prior therapy with imatinib, by both the Food and Drug Administration (FDA) in the United States and the European Agency for the Evaluation of Medicinal Products (EMEA) in Europe.
Acquired drug resistance to imatinib is usually associated with point mutations within the kinase domain of BCR-ABL. This acquired resistance led to the development of ATP-competitive small-molecule tyrosine kinase inhibitors with activity against clinically observed BCR-ABL mutants. At the present time, 2 such compounds have undergone phase 1 or 2 studies.2,3 Dasatinib and nilotinib were specifically designed to overcome imatinib resistance. While dasatinib binds to both active and inactive conformations of ABL kinase, nilotinib binds to the inactive ABL kinase. Compared with imatinib, dasatinib is at least 300-fold and nilotinib at least 20-fold more potent against unmutated ABL. Although they have similar efficacy, the toxicity profile is different, providing further rationale for the development of both compounds.
Many questions remain. Is there a role for dasatinib or nilotinib in newly-diagnosed CML patients? Could dasatinib or nilotinib suppress the emergence of resistant mutations? Is the response duration likely to be similar to that of imatinib? Since toxicity remains an important issue, could either of these agents be combined at lower doses when toxicity precludes the use of either compound in standard dose? Finally, overcoming the T315I mutation remains elusive. Developing such an inhibitor is the major challenge before the molecular gap is closed in CML.
The author declares no conflicting financial interests. ▪