Abstract
Leukocyte adhesion deficiency type 1 (LAD-1) is an autosomal recessive disorder caused by mutations in the ITGB2 (CD18) gene and characterized by recurrent severe infections, impaired pus formation, and defective wound healing. We describe an unusual case of severe phenotypic LAD-1 presenting with somatic mosaicism. The patient is a compound heterozygote bearing 2 different frameshift mutations that abrogate protein expression. However, CD18 expression was detected in a small proportion of T cells but was undetectable in granulocytes, monocytes, B cells, and natural killer (NK) cells. The T cells were not of maternal origin, lacked the paternal mutation, and showed a selective advantage in vivo. Molecular analysis using sorted CD18+ cells revealed them to be derived from a single CD8+ T cell carrying T-cell receptor VB22. These findings suggest that spontaneous in vivo reversion was responsible for the somatic mosaicism in our patient.
Introduction
Leukocyte adhesion deficiency type 1 (LAD-1) is an autosomal recessive immunodeficiency disorder characterized by recurrent bacterial infections, impaired pus formation, and defective wound healing.1,2 The disease gene, ITGB2, is located on 21q22.3 and encodes the common chain of the β2 integrin family, CD18.1,2 Integrins are cell membrane receptors composed of α and β subunits that mediate adhesion events in all tissues, and β2 integrins are expressed only on leukocytes as heterodimers with an α chain (CD11a/CD18, CD11b/CD18, CD11c/CD18). Patients with LAD-1 are therefore uniformly deficient in the expression of all 3 leukocyte integrins.1,2
Somatic mosaicism as a result of in vivo reversion has recently been increasingly reported in patients affected with primary immunodeficiencies.3 The resultant effects on clinical phenotype range from milder manifestations characterized as natural gene therapy to none.3 Thus, the potential clinical consequences of revertant mosaicism remain difficult to predict. Here, we describe an unusual case of LAD-1 with a mosaic pattern of CD18 expression and elucidate the nature of the reversion and its effects on revertant cells and clinical features.
Patient, materials, and methods
Patient
The patient was the first child of nonconsanguineous, healthy Japanese parents. At 1 day of age he developed omphalitis as a result of group B streptococcus and was successfully treated with intravenous antibiotics. The umbilical cord was separated at 17 days. At 2 months of age he had an infected urachal cyst that required antibiotics and surgical excision. Because of delayed wound healing and persistent leukocytosis (22.5-48.2 × 109/L), he was referred to our hospital at age 3 months. Fluorescence-activated cell sorting (FACS) analysis demonstrated the absence of CD18 (Figure 1A) and its associated molecules CD11b and CD11c (data not shown) on his granulocytes and monocytes. On the basis of these findings a diagnosis of LAD-1 was made. Despite prophylactic antibiotics, recurrent perianal cellulitis was noted from 5 months of age. The patient has not received any blood product transfusion.
Molecular and cellular studies
CD18+ cells were enriched from peripheral blood mononuclear cells (PBMCs) using magnetic beads (BD PharMingen, San Diego, CA). Microsatellite polymorphic markers, including D9S1198, the human androgen receptor gene, and the heme oxygenase-1 promoter, were amplified with FAM-labeled specific primers4,5 and subjected to GeneScan analysis.6 Mutation analysis of the ITGB2 gene, FACS analysis of T-cell receptor (TCR) VB repertoire, and complementarity-determining region 3 (CDR3) spectratyping were performed as described elsewhere.7-9 CD8+ T cells were examined for CD69 and CD25 expression by 3-color FACS analysis after incubation for 16 hours with a combination of 5 μg/mL anti-CD2 monoclonal antibodies (mAbs; Beckman Coulter, Fullerton, CA), alone or together with 100 U/mL interleukin-2 or 5 μg/mL anti-CD28 mAb (BD PharMingen).10 Approval for the study was obtained from the Human Research Committee of Kanazawa University Graduate School of Medical Science, and informed consent was provided according to the Declaration of Helsinki.
Results and discussion
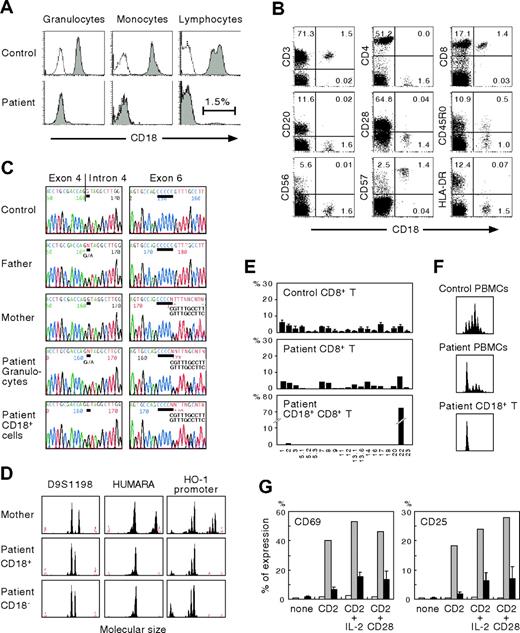
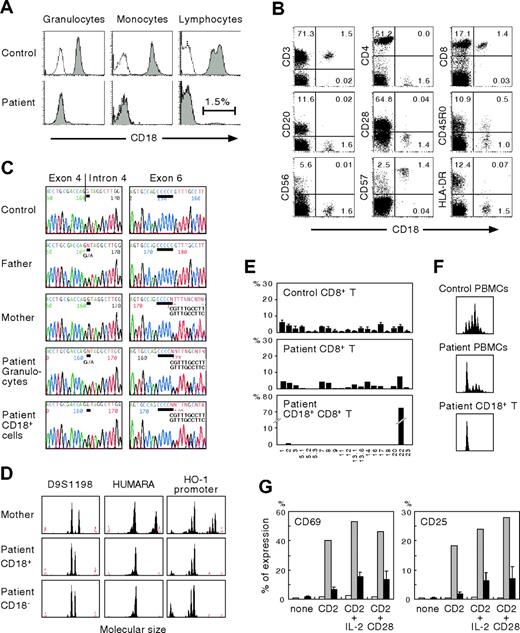
The clinical features of our patient were consistent with a severe form of LAD-1. Direct genomic sequence analysis of granulocytes showed him to be a compound heterozygote for 2 different mutations. One was a splice site mutation, G>A (+1), intron 4, that caused exon 4 skipping; the other was a single C deletion following nucleotides 670 to 674 in exon 6, both of which resulted in frameshift and affected the highly conserved domain (exons 5 through 9) of CD18 (Figure 1C). The parents were heterozygous carriers. These novel mutations should have led to the complete absence of biosynthesis of the β2 subunit of leukocyte integrins. However, a small proportion of the patient's lymphocytes were CD18+ (Figure 1A). To determine the lymphocyte population responsible for CD18 expression, multicolor FACS analysis was performed. As shown in Figure 1B, CD18+ cells were found to include CD8+ T cells but were not detected among CD4+ T, CD20+ B, or CD56+ natural killer (NK) cells. The CD18+CD8+ T cells expressed significant levels of CD57 but not CD28, indicating a memory/effector phenotype.
Characterization of CD18 expression, ITGB2 gene mutations, T-cell receptor repertoire, and T-cell functions. (A) Analysis of CD18 expression. Shown are the results of CD18 expression on granulocytes, monocytes, and lymphocytes. Open peaks indicate control Ab; solid peaks represent anti-CD18 mAb. (B) CD18 expression on the patient's lymphocyte subsets. The percentage of cells gated in each quadrant is shown. (C) Mutation analysis. The ITGB2 gene was amplified from DNA extracted from normal PBMCs, PBMCs from the parents, and the patient's granulocytes and CD18+ T cells. Direct sequencing was performed using an automated sequencer. Bars show the locations of the mutations. (D) Microsatellite analysis. Three different markers were amplified with FAM-labeled specific primers and subjected to GeneScan analysis. HUMARA indicates human androgen receptor gene; HO-1, heme oxygenase-1. (E) Expression profiles of TCR VB subfamilies. Peripheral blood samples were stained with mAbs for individual TCR VB subfamilies together with anti-CD8 and anti-CD18 mAbs. The expression of each TCR VB by CD8+ or CD18+CD8+ T cells was analyzed by FACS. (F) CDR3 spectratyping. A TCR VB22 fragment was amplified from cDNA with specific primers. The size distribution of polymerase chain reaction products was determined by GeneScan analysis. (G) Analysis of CD2-mediated T-cell activation. PBMCs from healthy control subjects and the patient were cultured for 16 hours with a combination of anti-CD2 mAbs alone or together with interleukin-2 or anti-CD28 mAb. CD69 and CD25 expression was evaluated in the patient's CD18−CD8+ T (□) and CD18+CD8+ T (⊡) cells. ▪ indicate control CD8+ T cells from healthy adults. Error bars represent SD.
Characterization of CD18 expression, ITGB2 gene mutations, T-cell receptor repertoire, and T-cell functions. (A) Analysis of CD18 expression. Shown are the results of CD18 expression on granulocytes, monocytes, and lymphocytes. Open peaks indicate control Ab; solid peaks represent anti-CD18 mAb. (B) CD18 expression on the patient's lymphocyte subsets. The percentage of cells gated in each quadrant is shown. (C) Mutation analysis. The ITGB2 gene was amplified from DNA extracted from normal PBMCs, PBMCs from the parents, and the patient's granulocytes and CD18+ T cells. Direct sequencing was performed using an automated sequencer. Bars show the locations of the mutations. (D) Microsatellite analysis. Three different markers were amplified with FAM-labeled specific primers and subjected to GeneScan analysis. HUMARA indicates human androgen receptor gene; HO-1, heme oxygenase-1. (E) Expression profiles of TCR VB subfamilies. Peripheral blood samples were stained with mAbs for individual TCR VB subfamilies together with anti-CD8 and anti-CD18 mAbs. The expression of each TCR VB by CD8+ or CD18+CD8+ T cells was analyzed by FACS. (F) CDR3 spectratyping. A TCR VB22 fragment was amplified from cDNA with specific primers. The size distribution of polymerase chain reaction products was determined by GeneScan analysis. (G) Analysis of CD2-mediated T-cell activation. PBMCs from healthy control subjects and the patient were cultured for 16 hours with a combination of anti-CD2 mAbs alone or together with interleukin-2 or anti-CD28 mAb. CD69 and CD25 expression was evaluated in the patient's CD18−CD8+ T (□) and CD18+CD8+ T (⊡) cells. ▪ indicate control CD8+ T cells from healthy adults. Error bars represent SD.
To assess whether the same mutations were present in the patient's CD18+ T cells, sequencing analysis was performed on genomic DNA obtained from enriched CD18+ T cells. The purity of the enriched cells was about 80%, as determined by FACS analysis. As shown in Figure 1C, we found that the patient's CD18+ T cells lacked the splice site mutation in intron 4, although a minor peak of nucleotide A originating from contaminating CD18− cells was also seen at the position of the mutation. Molecular typing of the enriched CD18+ T cells using 3 different polymorphic markers showed that they were not derived from maternal T-cell engraftment (Figure 1D). We therefore conclude that the patient's CD18+ T cells result from site-specific single nucleotide reversion of the inherited paternal mutation.
FACS analysis of the TCR VB repertoire clearly demonstrated that the patient's CD18+ T cells were detectable only with TCR VB22 (Figure 1E). Analysis of CDR3 spectratyping and the junctional amino acid sequence of TCR VB22 also showed a monoclonal CD18+ T-cell profile (Figure 1F; Table 1) Taken together, these findings suggest that the reversion of the paternal mutation occurred in a committed CD8+ T cell in the patient and that mature hematopoietic cells as well as early progenitors3 could be the elements in which the in vivo reversion occurred. In addition, the accumulation of CD18+ T cells to levels that reached the detection threshold of FACS analysis suggests that CD18+ T cells might have a selective growth advantage in vivo over CD18− cells. Indeed, in canine LAD models, following nonmyeloablative hematopoietic stem cell transplantation, the extent of donor chimerism was greater in peripheral T cells than in neutrophils and monocytes and increased progressively over time.11
T-cell activation mediated by a combination of anti-CD2 mAbs that react with T11.1 or T11.2 epitopes is severely defective in patients with LAD.10 To evaluate the functionality of CD18+CD8+ T cells we examined the surface expression of activation markers after incubation with anti-CD2 mAbs. As shown in Figure 1G, CD69 and CD25 expression on the patient's CD18−CD8+ T cells was impaired. In contrast, the revertant CD18+CD8+ T cells showed significantly improved responses to anti-CD2 stimulation, indicating that the genetic reversion had led to reversal of the biologic defects characteristic of LAD T cells. The severity of clinical features in patients with LAD-1 is directly related to the degree of CD18 deficiency. Patients with less than 1% residual expression of β2 integrins have the severe form of LAD-1, whereas those with 1% to 10% expression show a moderate phenotype.1 Our patient, however, has shown no improvement in clinical symptoms as a consequence of the revertant mosaicism, probably because no reversion events occurred in myeloid cells. These findings and previous observations6,9 suggest that the clinical consequences of reversion may depend on cell lineage, size and diversity, and the functional restoration of revertant cells.
In summary, our results provide an additional example of genetic reversion in a primary immunodeficiency and further support the possibility that somatic revertant mosaicism could be present in any genetic disorder in which revertant cells have a selective growth advantage in vivo but remain undetected because they do not necessarily result in modification of the clinical phenotype.
Authorship
Contribution: Y.T., T.W., and A.Y. participated in designing and performing the research; F.S., T.T., Y.H., Y.K., and S.K. analyzed the data; Y.T. and T.W. wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taizo Wada, Department of Pediatrics, Graduate School of Medical Science, Kanazawa University, 13-1 Takaramachi, Kanazawa 920-8641, Japan; e-mail: taizo@ped.m.kanazawa-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Harumi Matsukawa and Ms Mika Tamamura for excellent technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and a grant from the Ministry of Health, Labour, and Welfare of Japan, Tokyo.