Abstract
This study was set up to demonstrate whether prognostic classification based on the secondary age-adjusted International Prognostic Index (sAA-IPI) for recurring aggressive non-Hodgkin lymphoma (NHL) or the prognostic score for recurring Hodgkin lymphoma (HL) can be improved by including the midtreatment results of fluorine-18-fluorodeoxy-glucose–positron emission tomography (FDG-PET). Clinical data on patients with recurring lymphoma who were treated with second-line chemotherapy (DHAP-VIM-DHAP) followed by autologous stem cell transplantation (ASCT) were collected and combined with the results of FDG-PET performed before and after 2 cycles of reinduction chemotherapy. PET responses after 2 courses were scored as complete remission (CR), partial remission (PR), or no response (NR). A multivariate analysis was performed to design a predictive model. The number of patients (101 of 117) included those (78 patients with aggressive NHL and 23 patients with HL) that could be analyzed according to protocol. Of these, 80 patients were chemosensitive and 77 received transplants. Both secondary clinical risk score (P < .001) and FDG-PET response (P < .001) were independent predictive factors for the total evaluable group of patients with lymphoma and for patients with NHL alone. The combined use of the clinical risk score and FDG-PET response after 2 chemotherapy courses identified at least 4 categories of patients with a failure-free survival varying between 5% to 100% after transplantation (P < .001). These data indicate that the secondary clinical risk score in conjunction with FDG-PET response provides a more accurate prognostic instrument for the outcome of second-line treatment at least in patients with recurring NHL.
Introduction
Patients with a recurrence of aggressive non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) can be offered intensive chemotherapy followed by myeloablative therapy and autologous stem cell transplantation (ASCT). Especially in NHL, this treatment will only be successful in patients who still have chemosensitive disease.1,2 So, patients are selected for ASCT only if at least partial remission has been obtained confirmed by computed tomography (CT) or other conventional diagnostic methods. Despite this selection, 20% to 40% of the patients relapse after ASCT.1
In the last decade, fluorine-18-fluorodeoxy-glucose–positron emission tomography (FDG-PET) has been shown to be a useful tool to assess lymphoma activity before and after therapy. FDG-PET is more accurate in showing residual disease and predicting outcome after treatment than CT. Pretransplantation FDG-PET is predictive for outcome in patients with recurring lymphoma.3-5
Several clinical risk scores have been developed in primary untreated lymphoma, including the International Prognostic Index (IPI).6 Also, in relapsed lymphoma, prognosis is determined by a number of clinical and biological parameters. Hamlin et al7 validated a clinical score derived from the IPI for primary NHL, the secondary age-adjusted IPI (sAA-IPI). In 150 patients with recurring or primary refractory diffuse large-cell lymphoma treated with intensive chemotherapy and ASCT, it appeared that increased lactate dehydrogenase (LDH), advanced clinical stage, and impaired performance could predict unfavorable outcome. Likewise, a recent German study proposed a prognostic score for recurring Hodgkin lymphoma (rHPS) based on a group of 422 patients, of whom 140 were treated with intensive chemotherapy and ASCT.8 In this group, duration of first remission, clinical stage at relapse, and anemia were independent prognostic factors. Although these clinical risk scores are useful to determine the prognosis of patients with recurring lymphoma, they are not accurate enough to direct individual treatment decisions. In this study we questioned whether the addition of FDG-PET results to these clinical prognostic scores could improve patient selection for ASCT.
Patients, materials, and methods
Patients and treatment
Patients with histologically proven relapse or progression of either aggressive NHL or HL who were intended to be treated with second-line chemotherapy followed by myeloablative therapy and ASCT were eligible for this study. Between 1999 and 2004, patients from 2 hematologic centers (University Medical Center Groningen [UMCG], The Netherlands; and Vrije University Medical Center [VUMC], Amsterdam, The Netherlands) were prospectively included. A subset of this population has been described earlier.5,9 All patients were treated according to a standard treatment protocol consisting of DHAP (dexamethasone, cytarabine, and cisplatin), VIM (etoposide, iphosphamide, and methotrexate), and a second (mobilization) cycle of DHAP when chemoresponsive.10 A number (38) of patients with NHL also received rituximab during induction treatment. In total, 27 patients participated in the HOVON 44 trial comparing the addition of rituximab to standard treatment of relapsing B-cell NHL.
Chemoresponsiveness was based on conventional diagnostic methods, including physical examination, CT, and bone marrow biopsy (Cheson criteria) and defined as complete or partial remission.11 Two weeks after stem cell mobilization, patients were readmitted to receive myeloablative therapy consisting of BEAM (carmustine, etoposide, cytarabine, and melphalan) followed by ASCT.10 Nonresponding patients were offered salvage therapy consisting of mini-BEAM.12 When responsive, these patients received ASCT as well. Radiotherapy after transplantation could be applied in case of bulky disease. This was performed in 18 patients. All patients had a follow-up of at least 6 months after ASCT. The database for this analysis was closed in January 2005. A reference pathologist confirmed the histology of all biopsies. All included patients gave informed consent, and the medical ethics committee of our hospitals approved the protocol.
Clinical risk scores
The sAA-IPI is a clinical risk score evaluated for patients with recurring aggressive NHL.7 One point is counted for each of the following items: LDH above the upper limit of normal, Ann Arbor stage III or IV, and a WHO performance status of 2 or higher. Risk groups are defined as low (0 points), low-intermediate (1 point), high-intermediate (2 points), and high (3 points).
The recurring Hodgkin score (rHPS) is a clinical risk score evaluated for patients with recurring Hodgkin disease.8 One point is counted for each of the following items: duration of first remission less than 12 months, Ann Arbor stage III or IV, and hemoglobin levels of 120 g/L (12 g/dL) or less. Risk groups are defined as low (0 points), low-intermediate (1 point), high-intermediate (2 points), and high (3 points).
PET
Whole-body FDG-PET was performed before the start of reinduction treatment (PET1) and after DHAP and VIM (PET2).5 The patients received approximately 5 MBq/kg body weight FDG intravenously and were scanned from the midthigh to the crown upwards, starting 90 minutes after injection. Time per bed position was 8 minutes. Interleaved protocol (ETTE) scans were used to correct for attenuation of the FDG signal in most patients.
We used 2 scanners with an axial field of view (FOV) of 10.8 cm and a 6-mm resolution (ECAT model 951/31; Siemens/CTI, Knoxville, TN) and 15.4 cm and 5-mm resolution (ECAT EXACT HR+; Siemens/CTI), respectively. Most patients were scanned on the latter machine. Data were reconstructed iteratively into coronal, sagittal, and transverse sections and a 3D rotating maximum intensity projection using standard ECAT software.
Two independent reviewers (J.P. and B.W.S.), blinded to clinical and radiologic data, evaluated all serial scans. The response was scored by comparing PET1 and PET2 using visual assessment. Complete remission (CR) was defined as complete disappearance of all previous lesions with abnormal uptake; partial remission (PR) was defined as only residual PET abnormalities at the site of previous lesions with an intensity above the background level; and no response (NR) was defined as no distinct change or even progression of volume or intensity of any former pathologic lesion. The PR assessment is in line with minimal residual uptake described by Mikhaeel et al.13 In case of a discrepancy in response score between the 2 observers, an independent panel of PET readers decided on the matter. In case of any new lesion, CT results were used to discriminate lymphoma from nonlymphoma lesions.
CT
CT scans were performed in parallel to FDG-PET scans (at diagnosis of recurrence/progression of lymphoma and after 2 courses of induction chemotherapy), allowing a maximal interval of 2 weeks between CT and PET scans. CT scanning was performed after oral and intravenous contrast. Slice thickness varied from 0.5 cm in the neck region to 1.0 cm in the thorax and abdomen.
The number of enlarged lymph nodes was counted and the diameter of the largest lesions was measured in 2 perpendicular dimensions. After restaging, remission status was assessed using standardized response criteria according to the International Working Group recommendations.11
Statistics
The aim of this study was to evaluate the value of sAA-IPI, rHPS, and FDG-PET in assessing 2-year failure-free survival (FFS) for all patients intended to be treated with second-line chemotherapy and myeloablative therapy followed by stem cell reinfusion on an intention-to-treat basis. FFS was calculated from the date of the first PET scan until the second failure. Second failure was defined as recurrence or progression of lymphoma on CT scan, death due to lymphoma, or toxic death due to therapy. Data were censored if patients were alive and free of disease at last follow-up. Differences in events between groups were analyzed with the Student t test. FFS was calculated using Kaplan-Meier analysis and comparison between groups was performed using a log-rank test. The predictive value of FDG-PET was determined by a Chi-square test. Multivariate regression analysis (Cox) was used to determine prognostic factors in a predictive model.14
A P value smaller than .05 was considered statistically significant. Data analysis was performed using the SPSS 12.0 software package (SPSS Inc, Chicago, IL).
Results
Patient characteristics
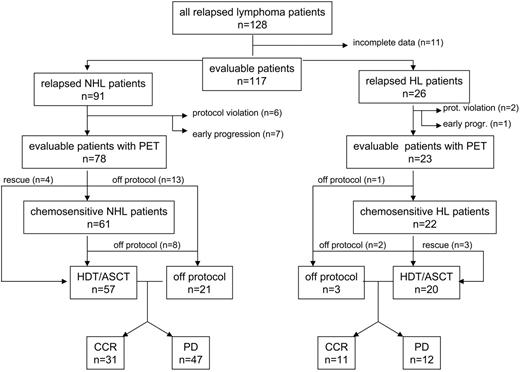
During the study period, 128 patients started reinduction treatment for relapse or progressive disease (Figure 1). A number (27) of patients could not be evaluated because of incomplete data (n = 11), missing PET scans due to early progression (n = 8), or protocol violation (n = 8). So, in total, 101 patients (78 with NHL and 23 with HL) were analyzed on the basis of the intention to proceed to ASCT (Table 1). Of these, 30 patients had primary refractory disease after first-line chemotherapy. Most patients with NHL had diffuse large B-cell lymphomas (68%) presenting with stages III to IV disease and with 2 to 3 risk factors. All patients were PET positive at first relapse. Patient characteristics are given in Table 1. In our analysis, median follow-up for nonrelapsing patients after ASCT was 22 months (range, 6-61 months).
Treatment and outcome
NHL.
Most (78) of the 91 patients with recurring NHL could be analyzed according to protocol.
After DHAP and VIM treatment, 61 of 78 patients with NHL were chemoresponsive and were treated with a second DHAP chemotherapy course followed by peripheral stem cell collection. Eight patients progressed shortly before ASCT and went off protocol. A number (17) of nonresponsive patients on DHAP-VIM received salvage chemotherapy. Four of these patients responded and underwent transplantation. In the end, 57 (73%) of 78 patients underwent transplantation. In total, 47 (60%) patients relapsed or progressed during follow-up, including patients who were not chemosensitive. These patients were offered palliative chemotherapy or radiotherapy. During the study period 39 (50%) patients died from progressive disease.
HL.
Most patients (23 of 26) could be analyzed according to protocol.
Of these, 22 of the patients with HL were chemoresponsive. The nonresponsive patient received salvage chemotherapy but progressed. Five chemosensitive patients progressed shortly before ASCT, of which 3 received rescue therapy and underwent transplantation. Finally, 20 (87%) of 23 patients with HL underwent transplantation. During follow-up, 12 (52%) patients relapsed, of which 11 patients died from progressive disease.
Clinical risk scores and outcome
NHL.
The sAA-IPI correlated well with the second relapse rate (Table 2).
Patients with a risk score of 0 or 1 had a relapse rate of 41% (11 of 27), while patients with a risk score of 2 or 3 had a relapse rate of 71% (36 of 51; P = .015). Based on survival analysis, the FFS was significantly different for each risk group. The 2-year FFS for risk scores 0 and 1 was 67% (95% confidence interval [CI], 48%-86%) and 56% (95% CI, 45%-67%), respectively, while the FFS for risk scores 2 and 3 was 26% (95% CI, 18%-34%) and 12% (95% CI, 1%-23%) (P = .018; Figure 2A). No significant difference in FFS was observed between the total group of patients with NHL (n = 91) and the PET-evaluable patients with NHL. When analyzing only those patients who underwent transplantation, the relapse rate (P = .122) and 2-year FFS (P = .085) were not significantly different for each risk group.
CliRS and FFS. CliRS and FFS are shown for 78 patients with recurring NHL (A) and 23 patients with HL (B). CliRS 0 indicates low risk; CliRS 1, low-intermediate risk; CliRS 2, high-intermediate risk; and CliRS 3, high risk.
CliRS and FFS. CliRS and FFS are shown for 78 patients with recurring NHL (A) and 23 patients with HL (B). CliRS 0 indicates low risk; CliRS 1, low-intermediate risk; CliRS 2, high-intermediate risk; and CliRS 3, high risk.
HL.
By using the rHPS it was demonstrated that the relapse rate increased with increasing score (Table 2).
Patients with risk scores 0 or 1 had a relapse rate of 44% (7 of 16 patients), while patients with a risk score of 2 or 3 had a relapse rate of 71% (5 of 7 patients; P = .37). The 2-year FFS for risk scores 0 and 1 was 75% (95% CI, 55%-95%) and 47% (95% CI, 32%-62%), respectively, while the FFS for risk scores 2 and 3 was 50% (95% CI, 30%-70%) and 0% (P = .57; Figure 2B). No difference in FFS was observed for the total group of patients with HL (n = 26) versus the PET-evaluable patients with HL (n = 23).
FDG-PET and outcome
NHL.
After DHAP-VIM treatment, 24% of the FDG-PET scans were normalized, 46% showed PR, and 29% showed no response (Table 3).
Nearly half (11 of 23) of the PET nonresponders went on to ASCT, of which 8 of 11 patients relapsed; 29 of 36 partial responders went on to ASCT, of which 15 relapsed; and 17 of 19 complete responders went on to ASCT, of which 3 relapsed. Two-year FFS was 72% (95% CI, 60%-82%) for patients with complete FDG-PET remission, 38% (95% CI, 30%-46%) for partial responders, and 10% (95% CI, 4%-16%) for nonresponders, respectively (P < .001; Figure 3A). When analyzing only those patients that underwent transplantation, relapse rate (P = .008) and 2-year FFS (P = .039) were significantly different for each risk group.
PET response and FFS. PETs and FFS are shown for 78 patients with recurring NHL (A) and 23 patients with recurring HL (B). Complete remission (CR), partial remission (PR), or nonresponse (NR) on FDG-PET after 2 cycles of induction chemotherapy.
PET response and FFS. PETs and FFS are shown for 78 patients with recurring NHL (A) and 23 patients with recurring HL (B). Complete remission (CR), partial remission (PR), or nonresponse (NR) on FDG-PET after 2 cycles of induction chemotherapy.
HL.
After DHAP-VIM treatment, 34% of the FDG-PET scans were negative, 43% showed PR, and 21% showed no response (Table 3).
All PET nonresponders went on to ASCT, after which 3 of 5 patients relapsed. Two-year FFS was 73% (95% CI, 57%-89%) for patients with CR at FDG-PET, 37% (95% CI, 21%-53%) for patients with a PR, and 40% (95% CI, 18%-62%) for the FDG-PET nonresponder group (not significant, P = .45; Figure 3B).
Univariate and multivariate analyses
In the univariate analysis the following items were scored: histology, LDH, clinical risk score, and FDG-PET response. In addition, primary refractory versus recurring disease was analyzed for patients with NHL only, as duration of first response is already included in the rHPS risk score.
A significant correlation was observed for LDH (P = .003), primary refractory disease (P < .001), clinical risk score (P < .001), and FDG-PET response (P < .001).
In the multivariate analysis, both clinical risk scores (P < .001) and FDG-PET (P < .001) are independent prognostic risk factors for progression (Table 4).
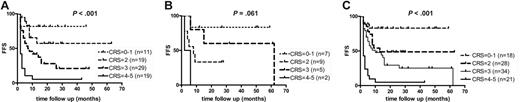
These findings were used to define a new risk-scoring system based on the relative contribution of both scoring systems. The points related to PET-CR(0) (0 points), PET-PR(1) (1 point), or PET-NR(2) (2 points) for FDG-PET response were combined with clinical risk score assessment (CliRS 0 indicates 0 points; CliRS 1, 1 point, etc). The sum of these points resulted in a combined risk score (CRS) of 6 different categories: CRS 0 to 5. Since only 4 patients had a score of 0 or 5, we decided to combine these into 4 well-balanced risk groups: CRS 0 to 1 (18 patients), CRS 2 (28 patients), CRS 3 (34 patients), and CRS 4 to 5 (21 patients) (Table 5). Table 5 shows how PET data add to the clinical risk score (eg, for those patients with higher risk scores, ASCT is less beneficial in patients with persisting PET activity). A high success rate (80%-100%) of ASCT could only be predicted if the combined risk score was 0 to 1 (n = 18). A low success rate (0%-7%) could be predicted if the combined risk score was 4 to 5 (n = 21).
The FFS of different risk groups according to their combined risk score is shown in a Kaplan-Meier curve (P < .001; Figure 4).
Kaplan-Meier curve showing FFS. FFSs are according to the combined use of the clinical risk score and the FDG-PET response in 78 patients with recurring NHL (A), 23 patients with recurring HL (B), and the total of 101 patients with recurring lymphoma (C). Four different categories of combined risk score (CRS) could be distinguished by the combined use of the clinical risk score and the FDG-PET response. The combined risk score is calculated by the sum of the clinical risk score (CliRS 0-3) and the PET response score (0-2).
Kaplan-Meier curve showing FFS. FFSs are according to the combined use of the clinical risk score and the FDG-PET response in 78 patients with recurring NHL (A), 23 patients with recurring HL (B), and the total of 101 patients with recurring lymphoma (C). Four different categories of combined risk score (CRS) could be distinguished by the combined use of the clinical risk score and the FDG-PET response. The combined risk score is calculated by the sum of the clinical risk score (CliRS 0-3) and the PET response score (0-2).
Discussion
Our multivariate analysis shows that both the clinical risk scores and early FDG-PET response are independent prognostic factors for outcome after second-line treatment consisting of reinduction chemotherapy and ASCT. The combined use might further improve the risk classification of patients with lymphoma failing first-line treatment and who are eligible for ASCT in order to stratify their treatment. In our analysis we used the recently described risk scores for recurring NHL and HL.7,8 The sAA-IPI has been validated in a large group of patients with recurring aggressive NHL treated with ICE (ifosfamide, carboplatin, and etoposide) and ASCT.7 For outcome in recurring HL, several risk factors have been described. Three risk factors were identified by Josting et al:8 time to relapse after first-line treatment, stage, and anemia. Other authors have found additional risk factors to be of importance in recurring HL, including age, albumin, lymphocytopenia, extranodal disease, and response characteristics after reinduction chemotherapy.15-18 In the present analysis we used risk scores of disease characteristics present at time of relapse. Both scoring systems for NHL and HL have a comparable design, run from 0 to 3 points, and appear to be very useful to predict outcome after ASCT. Duration of first response is an independent prognostic factor in HL and NHL. This parameter is included in the clinical risk score for HL. Although not included in the sAA-IPI, patients with primary refractory NHL have inferior outcomes compared with that of patients who relapsed, but the most important predictor for outcome is chemosensitivity.19 Response to the first-line therapy has no significant impact for outcome in the subgroup of chemosensitive patients who relapsed.7 Thus, although NHL and HL are 2 distinct entities, both diseases were combined in our model because the prognostic risk score for each disease was categorized according to a validated scoring system. This was further supported by the facts that histology was not a significant factor in the univariate analysis and that all patients were treated with the same second line of chemotherapy, including ASCT. However, when the results of NHL and HL are studied separately, no significance is observed for HL due to the limited number of patients.
Using a baseline and a midtreatment FDG-PET, we showed that FDG-PET response is an adequate parameter for chemosensitivity, but not for patients with progressive disease during the first courses of chemotherapy, which is less than 10% of the patients. Several studies have shown the superiority of FDG-PET over CT.20,21 In addition, the combined use of a baseline and midtreatment FDG-PET provides the opportunity to obtain a more accurate assessment of the FDG-PET response compared with a single midtreatment FDG-PET. A single midtreatment FDG-PET can only be positive or negative, while in the present study a distinction can be made between CR, PR, or NR. False-positive PET scans might be due to intercurrent inflammatory events giving rise to increased uptake of FDG. Since we compared the midtreatment scan with a pretreatment scan, knowledge of sites initially involved by lymphoma helped to reduce the number of false-positive scans. Five patients without PET response after induction treatment did not relapse during follow-up. These scans may have been false positives due to factors that are not related to lymphoma activity.
In the present study visual analysis was used for response measurement of FDG-PET. This appears to be an adequate method and is not inferior compared with standardized uptake value (SUV) analysis.9 In general, the disadvantage of SUV assessment is the diversity in acquisition and reconstruction parameters, and the correction factors that differ between PET centers. Especially for multicenter PET studies, it is attractive to use a visual assessment. In the present study the clinical risk score and FDG-PET results were independent prognostic factors. In contrast, Spaepen et al3 demonstrated the superiority of FDG-PET over the sAA-IPI. This discrepancy is most likely linked to differences in the composition of the studied group. For instance, the present study included more patients with aggressive NHL (78 of 101 patients in the present study vs 41 of 60 in the study by Spaepen et al). In addition, a CR on the FDG-PET was only observed in 24% of the patients in the present study, while Spaepen et al demonstrated a CR rate of 50% with the same chemotherapy regimen. The results of the present study are in line with the recently published data of Juweid et al.22 They demonstrated that the combined use of the International Workshop Criteria (IWC) plus FDG-PET assessment provides a more accurate response classification compared with the IWC alone in the upfront treatment of patients with aggressive NHL.
By using a FDG-PET scoring system running from 0 to 2 points for CR(0), PR(1), or NR(2), respectively, it was possible to develop a risk model for the combined use of the clinical risk score and FDG-PET response. The system provides a more accurate response classification for subgroups of patients with recurring NHL and possibly for patients with recurring HL, which might be used to develop alternative treatment strategies for poor-risk groups. The usefulness of this scoring system with its limitations has to be validated in a separate group of patients.
In conclusion, the present study demonstrates that the combined use of the clinical risk score and FDG-PET response after 2 courses of chemotherapy results in a more accurate prognostic classification at least for patients with NHL failing first-line treatment who are eligible for reinduction chemotherapy and ASCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: B.W.S. collected data, reviewed PET data, and wrote the article; J.M.Z. collected data and wrote the article; W.J.S. performed the statistical evaluation and created the prognostic model; G.W.v.I. reviewed the article; J.P. reviewed PET data and the article; and W.V. and E.V. reviewed the article and provided scientific support.
Acknowledgment
This work was supported by the University Medical Center Groningen.