We are in agreement that the role of surrogate markers in the treatment of Gaucher disease requires further critical examination. We also agree with Hollak et al that there are several aspects of serum chitotriosidase levels that make this parameter a promising surrogate for disease severity. The high levels found in Gaucher disease and the fact that Gaucher cells secrete the enzyme make chitotriosidase an excellent candidate for a useful biomarker. However, the “it stands to reason” approach often does not work out in medicine, and it is necessary for us to depend upon hard evidence to decide how useful a given biomarker is as a surrogate for disease severity. As we pointed out in our commentary,1 the published data are far from encouraging in this regard. Neither our own experience nor the published work of Deegan et al2 suggest that, in reality, chitotriosidase levels strongly reflect clinical disease severity. The data available from our patients at the Shaare Zedek Gaucher clinic are limited by the fact that chitotriosidase genotypes were not available on all patients and because we could include only those who had not received enzyme therapy before the levels of the enzyme were measured.
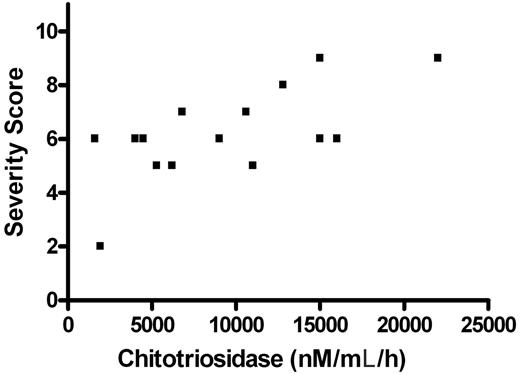
But the relationship between severity and enzyme level is very weak in those who could be included (Figure 1), and the average enzyme activity of the 2 patients with bone lesions averaged 6740, compared with an average of 8701 nM/mL per hour for the patients without bone lesions. Hollak et al again cite their earlier work with Maas et al4 that the fat fraction is a good surrogate for severity of bone disease. We reproduced Figure 3 from this paper in our commentary1 to show how weak the correlation was. Their study shows that 7 of 9 patients with mild or severe bone complications had fat fractions of less than 0.23, an arbitrary cutoff based on the data. But it also shows that 11 of 21 patients without bone complications had fat fractions below this arbitrary cutoff.4
The relationship between severity score3 and pretreatment chitotriosidase activity of 15 Gaucher disease patients seen at the Shaare Zedek Gaucher Clinic. All patients in whom plasma chitotriosidase levels before treatment and chitotriosidase genotypes were available are included except for a patient with the homozygous null mutation. The activity of 2 heterozygotes was multiplied by 2, as is the usual practice. While a correlation is found, it is so weak that we would not want to base a $300 000-a-year treatment decision on this relationship.
The relationship between severity score3 and pretreatment chitotriosidase activity of 15 Gaucher disease patients seen at the Shaare Zedek Gaucher Clinic. All patients in whom plasma chitotriosidase levels before treatment and chitotriosidase genotypes were available are included except for a patient with the homozygous null mutation. The activity of 2 heterozygotes was multiplied by 2, as is the usual practice. While a correlation is found, it is so weak that we would not want to base a $300 000-a-year treatment decision on this relationship.
Finally, while we agree that more work needs to be done to establish a role of surrogates in the treatment of Gaucher disease, the results with the parameters that gave “superior results” with high-dose therapy are not promising. These are slender reeds, indeed, upon which to make a $300 000-a-year treatment decision.
Conflict-of-interest disclosure: A.Z. and D.E. are consultants to Shire and are recipients of grants from Genzyme, for the International Collaborative Gaucher Group. A.Z. is also a consultant to Protalix Corporation.