Abstract
Mutations decreasing function of the Fas death receptor cause the autoimmune lymphoproliferative syndrome (ALPS) with autoimmune manifestations, spleen/lymph node enlargement, and expansion of CD4/CD8-negative T cells. Dianzani Autoimmune Lymphoproliferative Disease (DALD) is a variant lacking this expansion. Perforin is involved in cell-mediated cytotoxicity and its biallelic mutations cause familial hemophagocytic lymphohistiocytosis (HLH). We previously described an ALPS patient carrying heterozygous mutations of the Fas and perforin genes and suggested that they concurred in ALPS. This work extends the analysis to 14 ALPS, 28 DALD, and 816 controls, and detects an N252S amino acid substitution in 2 ALPS, and an A91V amino acid substitution in 6 DALD. N252S conferred an OR = 62.7 (P = .0016) for ALPS and A91V conferred an OR = 3 (P = .016) for DALD. Copresence of A91V and variations of the osteopontin gene previously associated with DALD conferred an OR = 17 (P = .0007) for DALD. In one N252S patient, NK activity was strikingly defective in early childhood, but became normal in late childhood. A91V patients displayed lower NK activity than controls. These data suggest that perforin variations are a susceptibility factor for ALPS/DALD development in subjects with defective Fas function and may influence disease expression.
Introduction
Fas is a death receptor belonging to the tumor necrosis factor receptor (TNFR) superfamily and induces cell death upon triggering by FasL.1-3 In the immune response, it is highly expressed by activated effector lymphocytes and is involved in switching off the immune response, limiting clonal expansion of lymphocytes, and favoring peripheral tolerance. Moreover, FasL is expressed by cytotoxic T cells and NK cells and is involved in killing of target cells expressing Fas. Fas induces cell apoptosis by triggering a cascade of caspases through 2 partly interconnected pathways: the extrinsic pathway involves caspase-8–mediated direct activation of the cascade, whereas the intrinsic pathway proceeds through mitochondrial release of cytochrome c and activation of caspase-9. Both pathways converge in the activation of effector caspases, such as caspase-3, -6, and -7.1-3
Defective Fas function leads to the unwanted accumulation of lymphocytes and favors autoimmunity possibly by impairing the switching off of autoreactive lymphocytes. This has been shown in the autoimmune lymphoproliferative syndrome (ALPS), an inherited disease characterized by (1) defective function of Fas, (2) autoimmune manifestations that predominantly involve blood cells, (3) polyclonal accumulation of lymphocytes in the spleen and lymph nodes with lymphoadenomegaly and/or splenomegaly, and (4) expansion of TCRαβ + CD4/CD8 double-negative (DN) T cells in the peripheral blood. Moreover, ALPS patients are predisposed to develop lymphomas in adulthood.3-11 ALPS is generally due to deleterious mutations of the Fas gene (TNFRSF6) and is classified as ALPS type-Ia, but rare mutations of other genes have been detected, for instance, the FasL genes in ALPS-Ib, and the caspase-10 gene in ALPS type-IIa, whereas the mutated gene is not known in other patients. Mutations of the Fas and the FasL gene detected in MLR lpr/lpr and gld/gld mice, respectively, give rise to a disease that overlaps ALPS. We described an ALPS variant that fulfils the first 3 criteria but lacks expansion of DN T cells and mutations of the Fas, FasL, or caspase-10 genes.12,13 Since the complete paradigm of ALPS could not be demonstrated, this disease has been provisionally named Dianzani Autoimmune Lymphoproliferative Disease (DALD) by McKusick (OMIM reference #605233; http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM).
ALPS-like disorders do not behave as classic monogenic diseases.3-8 This is true in lpr/lpr and gld/gld mice and even more evident in ALPS and DALD. The lpr and gld mutations cause the disease in homozygosity, but its expression greatly depends on the genetic background, since it is much milder in BALB/c than in MLR mice. Most ALPS type-Ia patients are heterozygous for the Fas mutation, but the parent carrying the mutation is generally healthy. Other complementary factors may thus be required in function of the severity of the mutation.14 The same observation is true in DALD patients, since both parents generally display defective Fas function, but are healthy.13 We suggested that a concurrent factor may be production of high levels of osteopontin (OPN), a cytokine involved in inflammation that inhibits activation-induced cell death (AICD) of lymphocytes. We found that DALD patients display increased frequency of polymorphic variants of the OPN gene (OPNhigh gene variants) that cause increased production of OPN by stabilizing its mRNA and increase the risk of DALD by about 8-fold.15,16
A second concurrent factor may be inherited alterations of the perforin gene (PRF1) that decrease the function of this protein, which is stored in the lytic granules of cytotoxic cells and plays a crucial role in cell-mediated cytotoxicity by forming pores in the target-cell membrane.17 Biallelic mutations of PRF1 cause about 30% of cases of familial hemophagocytic lymphohistiocytosis (HLH), a rare life-threatening immune deficiency ascribed to decreased capacity of CD8+ T cells and NK cells to kill virus-infected cells.17-22 A further 25% of patients display mutations of the MUNC 13-4 gene involved in perforin storage in the lytic granules and exocytosis.23 HLH is a recessive disease and subjects carrying heterozygous PRF1 mutations are generally healthy.
We have identified a heterozygous mutation of PRF1 in an ALPS patient who also carried a heterozygous mutation of the Fas gene. Since these mutations were inherited from distinct parents who were healthy, we suggested that their cotransmission was responsible for the son's ALPS.24
Systematic evaluation of the role of PRF1 in ALPS was not undertaken on that occasion. The present study has thus been performed to extend the analysis of PRF1 to a larger number of patients and evaluate its role in the development of ALPS and DALD.
Patients, materials, and methods
Patients
We analyzed 14 ALPS and 28 DALD Italian patients (some have already been presented in Dianzani et al,12 Ramenghi et al,13 and Campagnoli et al25 ). Diagnosis of ALPS was based on the presence of all the following criteria: (1) autoimmune manifestations; (2) chronic nonmalignant lymphadenopathy (2 or more lymph nodes enlarged over 2 cm in diameter) and/or splenomegaly; (3) defective Fas-induced apoptosis in vitro; and (4) mutations in the Fas, FasL, or caspase-10 genes and/or expansion of DN T cells in the peripheral blood. The Fas, FasL, caspase-10, and OPN genes were sequenced from genomic DNA, as previously reported.12,13 Seven ALPS patients (patients 1-7) carried heterozygous mutations of the Fas gene.
Diagnosis of DALD was based on the presence of the first 3 criteria, but lack of the fourth one. Two DALD patients (DALD-2 and -24) carried a heterozygous variation of the caspase-10 gene, causing a V410I amino acid substitution, initially associated with ALPS in homozygosity,10 but then recognized as a polymorphism.25,26
No patients displayed the diagnostic criteria for HLH. Controls (n = 816) were ethnically matched, healthy individuals. All patients and controls were unrelated, white and Italian. Peripheral-blood specimens and serum were obtained from patients and healthy controls with written informed consent, which was obtained in accordance with the Declaration of Helsinki. The study was performed according to the guidelines of the local ethics committee of the Ospedale Maggiore of Novara (Novara, Italy).
Flow cytometry
Analysis of lymphocyte subpopulations in peripheral-blood mononuclear cells (PBMCs) was performed by direct immunofluorescence and flow cytometry. Perforin expression was evaluated in fixed and permeabilized cells (Cytofix-Cytoperm; BD PharMingen, San Diego, CA) using a phycoerythrin (PE)–conjugated antiperforin antibody (BD PharMingen) and flow cytometry.
Fas function assay
Fas-induced cell death was evaluated as previously reported on T-cell lines obtained by activating PBMCs with phytohemagglutinin at days 0 (1 μg/mL) and 15 (0.1 μg/mL) and cultured in RPMI 1640 + 10% fetal calf serum (FCS) + rIL-2 (2 U/mL) (Biogen, Geneva, Switzerland). Fas function was assessed 6 days after the second stimulation (day 21).12,13 Cells were incubated with control medium or anti-Fas MAb (CH11, IgM isotype) (1 μg/mL) (UBI, Lake Placid, NY) in the presence of rIL-2 (1 U/mL) to minimize spontaneous cell death. Cell survival was evaluated after 18 hours by counting live cells in each well by the trypan blue exclusion test and by flow cytometry of cells excluding propidium iodide and unstained by annexin V–FITC; the 2 methods gave overlapping results. Assays were performed in duplicate. Cells from 2 healthy donors were included in each experiment as positive controls. Results were expressed as specific cell-survival percent, calculated as follows: (total live-cell count in the assay well/total live-cell count in the control well) × 100.
Fas function was defined as defective when cell survival was less than 82% (the 95th percentile of data obtained from 200 healthy controls).
Amplification of PRF1 and mutation detection
Genomic DNA was isolated from PBMCs, and exon 2 and 3 of the perforin coding region were amplified in standard polymerase chain reaction (PCR) conditions. The primers used for amplification have been previously described. PCR products were purified with the EXO/SAP kit.24 Sequencing was performed with the ABI PRISMR BigDye Terminator kit (Applied Biosystems, Foster City, CA) on an automatic sequencer (Applied Biosystems 3100 Genetic Analyzer) according to the manufacturer's instructions with the amplification primers plus 2 internal primers (forward 5′-CAGGTCAACATAGGCATCCACG-3′; reverse 5′-GAACAGCAGGTCGTTAATGGAG-3′) for exon 3. OPN gene variants were typed as previously reported.15
Cytotoxicity assays
NK activity of PBMCs was assessed by a standard 4-hour 51Cr release assay with K562 cells as the target. Results are expressed as specific lysis percent calculated as follows: (sample 51Cr release-spontaneous release)/(maximal release-spontaneous release) × 100.
Statistical analysis
Comparisons of NK activity, perforin expression, and NK-cell distribution were performed with the nonparametric Mann-Whitney U test. Genotype distributions were analyzed with the χ2 test or the Fisher exact test as reported. All P values are 2-tailed, and the significance cut-off was P below .05.
Results
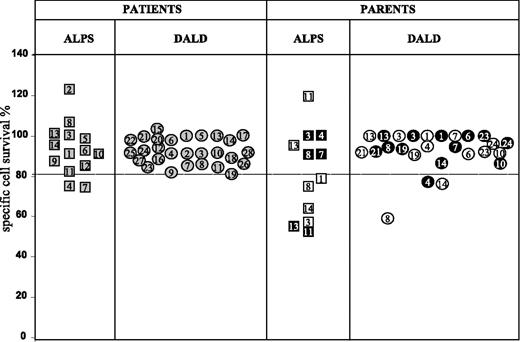
The work involved 14 patients with ALPS and 28 with DALD. Both groups displayed autoimmune manifestations, lymphadenopathy and/or splenomegaly, and defective Fas function; ALPS patients alone also displayed mutations of the Fas gene and/or peripheral-blood expansion of DN T cells. Figure 1 shows Fas function of T cells from all patients and available parents. That of ALPS-4 and -7 was borderline, but they were included in the ALPS group because they also carried a Fas gene mutation and expansion of DN T cells. Most parents, too, displayed defective Fas function.
The coding region of PRF1 was sequenced from genomic DNA in all patients and 816 random controls in the search for variations previously associated with HLH. Only 2 HLH-associated missense variations were detected, a C/T substitution in position 272 of the cDNA (numerations are referred to cDNA clone M28393, ATG =+1) and an A/G substitution in position 755, which caused an A91V and an N252S amino acid substitution at the protein level, respectively. The genotypic distributions of these variations did not deviate significantly from the Hardy-Weinberg equilibrium in either group.
Fas-induced T-cell death in patients with ALPS or DALD and several of their parents. Data from ALPS patients' families are marked with squares, those from DALD patients' families with circles; gray symbols mark patients; black symbols, the fathers; and white symbols, the mothers. Numbers correspond to the code assigned to each patient. Long-term T-cell lines were treated with anti-Fas Mab, and survival was assessed after 18 hours. Results are expressed as relative cell-survival percent. The horizontal lines indicate the upper limit of the normal range, calculated as the 95th percentile of data obtained from 200 healthy donors (median cell survival of controls was 60%; the 5th-95th percentile range was 38%-82%). In the control wells (ie, in the absence of apoptotic stimuli), spontaneous cell loss was always less than 10% of the seeded cells and similar in cultures from the patients and healthy donors. ALPS patients 1 to 7 carried heterozygous mutations of the Fas gene.
Fas-induced T-cell death in patients with ALPS or DALD and several of their parents. Data from ALPS patients' families are marked with squares, those from DALD patients' families with circles; gray symbols mark patients; black symbols, the fathers; and white symbols, the mothers. Numbers correspond to the code assigned to each patient. Long-term T-cell lines were treated with anti-Fas Mab, and survival was assessed after 18 hours. Results are expressed as relative cell-survival percent. The horizontal lines indicate the upper limit of the normal range, calculated as the 95th percentile of data obtained from 200 healthy donors (median cell survival of controls was 60%; the 5th-95th percentile range was 38%-82%). In the control wells (ie, in the absence of apoptotic stimuli), spontaneous cell loss was always less than 10% of the seeded cells and similar in cultures from the patients and healthy donors. ALPS patients 1 to 7 carried heterozygous mutations of the Fas gene.
The N252S substitution was found in 2 ALPS patients (ALPS-5 and ALPS-11), 2 controls, and no DALD patients. The overall genotype distributions (Table 1) were significantly different in ALPS and controls (P = .0016). The N252S allelic frequency was significantly higher in the ALPS patients (7.1% vs 0.1%, P = .0016) and conferred an OR = 62.7 (95% CI: 6-654.9). This variation had been previously reported by some of us in ALPS-5.24
The A91V variation was carried by 6 DALD patients only: it was heterozygous in 5 (DALD-3, -6, -9, -14, -25) and homozygous in one (DALD-10). Moreover, it was detected in 72 controls: heterozygous in 69 and homozygous in 3. The overall genotype distributions (Table 1) were significantly different in DALD and controls (P = .01). The A91V allelic frequency was significantly higher in the DALD patients (12.5% vs 4.6%, P = .016) and conferred an OR = 3 (95% CI: 1.2-7.1).
Four other nucleotide variations were detected, but were not further evaluated since they did not change the amino acid nor influence the splicing sites. Two (C822T and T900C) had been previously reported as common polymorphisms not associated with HLH. Their frequency was similar in the patients and the controls. The other 2 (G435A and A462G) were in perfect linkage disequilibrium with the A/G substitution in position 755 (N252S) and were in fact only detected in the 4 subjects carrying this variation.
We had previously found that DALD development is favored by the 282C-750T-1083A-1239C and 282C-750T-1083G-1239C single nucleotide polymorphism haplotypes of the OPN gene (OPNhigh gene variants).15 To determine whether PRF1 and OPN variations have a cooperative effect on ALPS/DALD development, we typed the OPN gene in all patients and 134 controls and evaluated the frequency of copresence of the PRF1 and OPN genotypes conferring susceptibility to ALPS/DALD (Table 1). Copresence was displayed by 6 (21.4%) of 28 DALD patients, but only 3 (2.2%) of 134 controls, and increased the risk of DALD by 17-fold relatively to the absence of both factors (OR = 17; 95% CI: 2.7-122; P = .0007) and by 9-fold relatively to the presence of only one (OR = 8.8, 95% CI: 1.7-50.5; P = .004). By contrast, this cooperation was not detected in ALPS patients since none of them carried both factors.
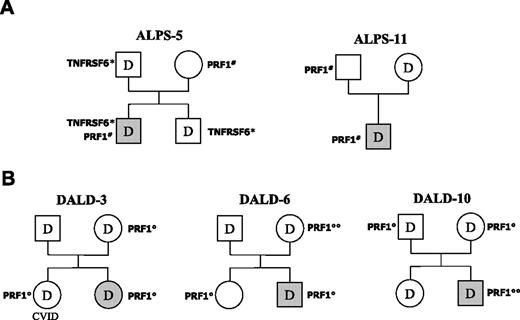
We had previously shown that ALPS-5 carried a heterozygous mutation of the Fas gene.24 His PRF1 N252S variation was inherited from the mother, whereas the Fas mutation also was carried by the father and a brother, and cosegregated with defective Fas function (Figure 2A). Since all 3 relatives were healthy, we suggested that co-inheritance of the Fas and perforin gene variations played a role in ALPS development in this patient. The mutated gene was not known in ALPS-11, and his inheritance pattern was determined by analyzing Fas function and sequencing PRF1 in his parents. N252S was carried by his father only, whereas Fas function was defective in his mother only (Figure 2A). Once again, therefore, Fas and perforin alterations were inherited from different parents, who were both healthy. These data indicate that association of defective Fas function with the N252S variation strongly favors ALPS development.
A family analysis also was conducted for 3 DALD patients carrying the A91V variation (DALD-3, -6, -10, Figure 2B). In DALD-3's family, Fas function was defective in both parents and the sister, whereas A91V was carried by the mother and the sister: both parents were healthy, whereas the sister presented common variable immune deficiency (CVID). Intriguingly, DALD-3 also developed hypogammaglobulinemia some years after disease onset. In DALD-6's family, Fas function was defective in both parents, whereas A91V was carried by the mother (homozygous) and the sister; all these relatives were healthy. In DALD-10's family, Fas function was defective in both parents and the sister, whereas A91V was carried by both parents; all these relatives were healthy. Analysis of the OPN gene showed that all subjects in these 3 families also carried the OPNhigh gene variants (data not shown). Association of defective Fas function and the A91V variation is thus not sufficient to induce DALD, even in the presence of the OPN susceptibility alleles, since 4 of 8 subjects with this association were healthy.
Pedigrees of patients ALPS-5 and -11 and DALD-3,-6, and -10. (A) Inheritance of the N252S PRF1 mutation (PRF1#) and defective Fas function (D) in ALPS-5 and -11; inheritance of the Fas mutation (TNFRSF6*) is also shown for ALPS-5. (B) Inheritance of the A91V PRF1 variation (PRF1°) and defective Fas function (D) in the 3 DALD patients. Subjects with ALPS/DALD are marked in gray; the sister of DALD-3 displayed CVID. PRF1°° marks A91V homozygotes. Fas function was evaluated as reported in Figure 1.
Pedigrees of patients ALPS-5 and -11 and DALD-3,-6, and -10. (A) Inheritance of the N252S PRF1 mutation (PRF1#) and defective Fas function (D) in ALPS-5 and -11; inheritance of the Fas mutation (TNFRSF6*) is also shown for ALPS-5. (B) Inheritance of the A91V PRF1 variation (PRF1°) and defective Fas function (D) in the 3 DALD patients. Subjects with ALPS/DALD are marked in gray; the sister of DALD-3 displayed CVID. PRF1°° marks A91V homozygotes. Fas function was evaluated as reported in Figure 1.
To assess whether N252S and A91V correlated with altered function and/or expression of perforin, NK activity was evaluated by a standard 51Cr-release assay and perforin expression by flow cytometry in 5 patients: ALPS-5 and -11 with N252S, and DALD-3, -6, and -10 with A91V. Perforin expression was slightly decreased in ALPS-5, DALD-6, and DALD-10, but normal in the other subjects, whereas the proportion of NK cells, detected as CD3–CD56+ or CD3–CD16+ cells, was in the normal range in all subjects. NK activity was significantly lower in the patients with A91V than in the controls (P = .015), but not decreased in those with N252S (Table 2). However, in ALPS-11, previous analyses showed that NK activity was almost undetectable at the age of 3 (ie, at diagnosis), extremely low but detectable at the age of 5, and normal at the age of 12 (Table 2). In ALPS-5, NK activity was assessed at the age of 30, and no previous analyses were available.
Discussion
This paper follows a description of an ALPS patient (ALPS-5) with variations of both the Fas gene and PRF1.24 It shows that his N252S variation is significantly more frequent in ALPS patients than in healthy controls. Moreover, the frequency of a second HLH-associated PRF1 variation, A91V, is significantly increased in DALD patients, who display an incomplete ALPS pattern. N252S was detected in 2 of 14 ALPS patients (ALPS-5 and -11). It increased susceptibility to ALPS by about 63-fold and was found only in 2 of 816 ethnically matched controls, as in other studies.27 N252S occurs within the membrane-attack-complex, a region critically involved in the pore-forming activity of perforin, but its functional significance has been debated since it has been associated with normal NK activity17,28,29 as in both ALPS-5 and -11 at the time of this study. The finding that ALPS-11 displayed a striking defect of NK activity when he was 3 and 5 years old suggests that N252S is here associated with other factors decreasing NK function in early childhood, followed by normalization on the part of unknown compensatory mechanisms.
A91V was detected in 6 of 28 DALD patients. It increased susceptibility to DALD by 3-fold and was relatively frequent (4.6%) in the controls, as in other studies.27,30,31 This variation decreases perforin function by altering its conformation, decreasing its cleavage to the active form and increasing its degradation.28-30 In line with this view, our patients displayed lower NK activity than the controls, especially at low effector-to-target ratios.
These data suggest that some PRF1 variations that cause HLH when combined with a second PRF1 variation may favor ALPS/DALD development if inherited defects hitting Fas function also are present. Fas function is normal in HLH and hence is not a contributory of this disease.21
The family analyses showed that combination of A91V with the Fas defect was not sufficient to induce DALD since several healthy family members carried both alterations. This risk was significantly increased by copresence of the OPNhigh gene variants, but even their combination with A91V and the Fas defect was not sufficient to induce DALD in 4 of 8 DALD family members. Combination of N252S with the Fas defect seems to have a stronger effect than A91V, since we found it in patients only. However, even this combination may not be sufficient for ALPS development, since Rieux-Laucat et al32 have described an ALPS patient and his healthy father carrying both N252S and a Fas gene mutation. The observation that N252S is in perfect linkage disequilibrium with G435A and A462G raises the possibility that these variations or others included in the ancestral haplotype play a role in ALPS development. A second possibility is that concurrent roles are played by other factors. The fact that OPN did not cooperate with N252S in ALPS development may be related to the stronger effect displayed by N252S in ALPS than by A91V in DALD, and to the possibility that the genetic hit of Fas function is more severe in ALPS than in DALD.
Fas and perforin alterations may cooperate in affecting both the antiviral response and the switching off of the immune response. Both molecules are used by cytotoxic cells to kill virus-infected cells. Moreover, Fas is highly expressed by effector lymphocytes that are switched off by several FasL+ cell types, but a regulatory activity also has been ascribed to perforin-mediated killing of effector lymphocytes and antigen-presenting cells.33-43 In this connection, it is noteworthy that OPN inhibits lymphocyte AICD, another mechanism involved in switching off the immune response. Lymphocyte accumulation and autoimmunity displayed by ALPS and DALD patients may be favored by both defective immune response switching off and decreased virus clearance that would prolong the immune response. This possibility opens the way to the view that ALPS/DALD may overlap both HLH and other inherited diseases characterized by lymphoproliferation and defective control of viral infections, such as the X-linked lymphoproliferative syndrome (XLP) due to mutations of the SAP gene altering function of the 2B4 NK coreceptor.44 Intriguingly, both ALPS and XLP are associated with high susceptibility to lymphoid neoplasia, which also seems favored by inherited PRF1 variations.45
Prepublished online as Blood First Edition Paper, May 23, 2006; DOI 10.1182/blood-2006-02-001412.
R.C. and A.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This work was partially supported by Telethon grant E1170 (Rome), Fondazione Italiana Sclerosi Multipla (FISM) grant 2003/R/20 (Genoa), Programma di Rilevante Interesse Nazionale (PRIN) Project (Ministero Istruzione Universita Ricerca [MIUR], Rome), Fondazione Cariplo (Milan), Compagnia di San Paolo (Turin), Regione Piemonte (Turin), and Associazione Italiana Ricerca sul Cancro (AIRC) (Milan).