Abstract
The expression of CD45RA on CCR7– human CD8+ memory T cells is widely considered to be a marker of terminal differentiation. We studied the time course of CD45RA and CCR7 expression on human antitumoral cytotoxic T lymphocyte (CTL) clones and blood CD8+ T cells after antigenic stimulation. Our results indicate that CD45RA+CCR7– CD8+ T cells are resting memory cells that, upon antigenic stimulation and during the next 10 days, proliferate, lose CD45RA, and transiently acquire CCR7. In the absence of further antigenic stimulation, they progressively re-express CD45RA and become CD45RA+CCR7–. We conclude that the expression of CD45RA on these cells is indicative of the time elapsed since the last antigenic stimulation rather than the incapacity to proliferate or particularly high lytic potential. This concept leads to a reinterpretation of the significance of the presence of CD45RA+ CD8+ memory cells in patients affected by viral infections or by cancer.
Introduction
The differentiation of naive T cells into memory and effector cells is marked by changes in the expression of surface molecules, such as CCR7 and CD45.1 Chemokine receptor CCR7 enables cells to migrate to lymph nodes.2,3 Naive T cells express the long CD45RA isoform of the cell-surface tyrosine phosphatase CD45. It was first thought that the expression of CD45RO, the short CD45 isoform, marks memory and effector T cells, but reversion from the CD45RO phenotype to the CD45RA phenotype was observed.4-6
Staining blood T cells with antibodies to CD45RA and CCR7 revealed 3 subsets of CD4+ T cells and Lanzavecchia and Sallusto proposed a linear differentiation model based on these 2 markers.7 In this model, the naive T cells are CD45RA+CCR7+. Upon antigen encounter, they lose CD45RA and differentiate first into central memory (TCM) cells, which are CD45RA–CCR7+. Upon further stimulation these cells differentiate into CD45RA–CCR7– effector memory (TEM) cells. It was proposed that the TCM subset is composed of partially differentiated lymph node–homing cells with a high proliferation capacity and production of IL-2 but little or no effector function, in contrast to the TEM cells, which migrate to inflamed tissues.1,7,8 Within the CD8+ T cells, the same 3 subsets were identified, plus an additional CD45RA+CCR7– subset of cells (TEMRA). These CD45RA+CCR7– CD8+ T cells were reported to have very little or no proliferative capacity, except in the presence of homeostatic cytokines IL-7 and IL-15, to be highly sensitive to apoptosis, to secrete IFN-γ but nearly no IL-2, and to express high perforin levels.9,10 Accordingly, the CD45RA+CCR7– T cells were presented as the ultimate stage of differentiation of memory CD8 T cells, and were named terminally differentiated effector cells, even though other studies indicated that this subset of CD8+ T cells could be quite heterogeneous for the expression of granzyme B, TNF-α, IFN-γ, and FasL.11 The quality of immune responses to viruses and tumors was often evaluated in the light of this terminal differentiation concept, and it was proposed that the CD45RA+CCR7– cells are the most effective CD8+ T cells for the destruction of tumor cells and virus-infected cells.
The absence of proliferative capacity of the CD45RA+CCR7– cells was challenged by reports on antiviral cytotoxic T lymphocytes (CTLs). The anticytomegalovirus (CMV) CTLs in the blood of a CMV healthy carrier could be identified with an A2/CMV tetramer and were found to have the CD45RA+CCR7– phenotype. Upon stimulation with a CMV peptide, these cells proved capable of proliferating at least 20-fold after 5 days.12 Very similar findings were made with Epstein-Barr virus (EBV). After resolution of mononucleosis, the CTLs directed against the BZLF1 protein of EBV had a CD45RA+CCR7– phenotype. When these CD8+CD45RA+ T cells were sorted and stimulated with a BZLF1 peptide, the anti-BZLF1 CTLs detected 7 days later by tetramer had also proliferated considerably.13 Moreover, both the anti-CMV and the anti-EBV CD45RA+ cells were found to revert to a CD45RA– phenotype following antigenic stimulation.
CCR7 was proposed as a marker that is irreversibly lost by the noneffector TCM cells differentiating into effector TEM cells.14 However, when blood cells from a human immunodeficiency virus (HIV)–infected subject that contain CCR7– anti-gag CTLs were stimulated with a gag peptide, a large fraction of the anti-gag CTLs re-expressed CCR7.10 The same observation was made for anti-CMV CTLs.15 When CD8+ CD45RA–CCR7– blood cells were stimulated with anti-CD3 and anti-CD28 antibodies, the re-expression of CCR7 was shown to be transient and to last up to 7 days.16
These observations have not led to a coherent view of the significance of the CD45RA and CCR7 expression on memory and effector CD8+ T cells, in the presence and in the absence of antigenic stimulation. We considered that the analysis of CTL clones might facilitate our understanding of the appearance and disappearance of the differentiation markers under these circumstances. We examined this issue with a set of CTL clones directed against a tumor-specific antigen encoded by gene MAGE-3.
Materials and methods
Anti–MAGE-3.A1 CTL clones
CTL clones were derived from anti–MAGE-3.A1 CTL precursors present in the blood of 3 different patients vaccinated with autologous mature dendritic cells pulsed with the peptide MAGE-3168-176 EVDPIGHLY presented by HLA-A1 molecules.17,18 The CTL clones were stimulated with irradiated (100 gray) HLA-A1+ MAGE-3+ MZ2-MEL.43 tumor cells pulsed for 1 hour with the MAGE-3 peptide (20 μM). Usually, CTL clones were cultured with the tumor cells at a 1:1 ratio in Iscove medium supplemented with 0.55 mM l-arginine, 0.24 mM l-asparagine, 1.5 mM l-glutamine, 50 μM β-mercaptoethanol (AAGβ), and 10% human serum. IL-2 (Chiron, Amsterdam, the Netherlands) was at 50 U/mL, and IL-7 and IL-15 (Peprotech, Rocky Hill, NJ) were at 10 ng/mL. For some experiments, irradiated HLA-A1 allogeneic Epstein-Barr virus–infected B (EBV-B) cells or irradiated HLA-A1 allogeneic mature dendritic cells, pulsed (or not) with the MAGE-3 peptide (20 μM), were used as stimulating cells. Dendritic cells were obtained from monocytes, matured as described earlier,19 and pulsed with the MAGE-3 peptide if required. When indicated, nonpulsed irradiated allogeneic EBV-B cells were added as feeder cells. For nonspecific TCR stimulation, CTLs were cultured with anti-CD3/CD28–coated beads (Dynal, Oslo, Norway) at a 1:3 ratio. If CTL cultures were maintained for more than 15 days without stimulation, half of the medium was replaced every 2 weeks with fresh medium and cytokines. The data shown in Figure 2C were obtained with clone DIPE6/8, an HLA-A2–restricted CD8+ CTL clone directed at an EBV-derived peptide, GLCTLVAML, encoded by gene BMLF1. This clone was obtained by limiting dilution from a population of cells stained with an A2/BMLF1 multimer.
Antibodies
Allophycocyanin (APC)–conjugated anti–human CD8 antibody (RPA-T8; BD Pharmingen, San Diego, CA), fluorescein (FITC)–conjugated anti–human CCR7 antibody (150503; R&D Systems, Abingdon, United Kingdom), phycoerythrin (PE)–conjugated anti–human CD45RA antibody (HI100; BD Pharmingen), and PE- or FITC-conjugated anti–human CD25 antibody (M-A251; BD Pharmingen) were used for T-cell phenotype characterization. The CTLs shown in Figure 6 were labeled with a nonlabeled anti–human CCR7 antibody (150503; R&D Systems), a biotin-conjugated rat anti–mouse IgG2a antibody (R19-15, BD Pharmingen), and a phycoerythrin-Cy5 (PE-Cy5)–conjugated streptavidin. The samples were analyzed by flow cytometry on a FACSCalibur using CellQuest software (Becton Dickinson, Mountain View, CA).
Evaluation of the expansion
Considering that many CTLs died within 2 days after TCR stimulation, the expansion of the clones was estimated in a flow cytometer by counting CD8+ cells in the cocultures at day 2 and day 5. Samples of the cocultures were labeled with APC-conjugated anti–human CD8 antibody; fluorescent beads (Fluoresbrite Plain YG 6 μm; Polysciences, Warrington, PA) were added in the sample at a known concentration in order to normalize the volume. The number of living CD8+ cells and the number of fluorescent beads acquired during the analysis were used to calculate the number of CD8+ cells in the coculture.
Lysis assay by flow cytometry
CTLs (20 000) were cocultured with 20 000 HLA-A1 EBV-B cells pulsed with MAGE-3 peptide (20 μM) in 200 μL medium. Three hours later, 70 μL of the cocultures was labeled with APC-conjugated anti–human CD8 antibody as a marker of the effector cells, peridinin chlorophyll protein–conjugated anti–human CD19 antibody (SJ25C1; BD Pharmingen) as a marker of the target cells, and propidium iodide as vital dye. Fluorescent beads were added to the coculture samples at a known concentration in order to normalize the volume. The number of living CD19+ target cells was evaluated by flow cytometry in the control sample containing only the targets and in duplicate cocultures.
Cytokine production
CTLs (20 000) were cocultured with 20 000 HLA-A1 EBV-B cells pulsed with MAGE-3 peptide at 20 μM in 200 μL Iscove medium supplemented with 1% human serum. Eighteen hours later, cytokine release was measured in the supernatant with the Cytometric Bead Array kit (CBA, Th1/Th2; BD Pharmingen).
Quantitative PCR
After stimulation of CTL clone BAGM9, samples were collected from the coculture at different time points. Total RNA was extracted with the PURESCRIPT Kit (Gentra, Minneapolis, MN) and converted to cDNA with M-murine leukemia virus reverse transcriptase using an OligodT (Life Technologies, Merelbeke, Belgium). Numbers of CCR7, CD45RA, and CD8β transcripts were evaluated by quantitative polymerase chain reaction (q-PCR) on an ICycler (Bio-Rad, Foster City, CA). For each gene, a standard curve was constructed as follows: a reference sample with a known number of cDNA copies of the relevant gene was diluted (10 ×, from 3 × 105 copies to 0.3 copy). The threshold cycles were determined for each dilution and used to build a standard curve. Threshold cycles were then calculated for each sample and were reported on the standard curve to estimate the absolute number of transcripts. The results for CCR7 and CD45RA were normalized to the number of CD8β transcripts. They were expressed in number of mRNA copies per CTL, on the basis of one CTL containing 40 CD8β transcripts. This estimate of 40 CD8β mRNA per cell was based on observations with peripheral-blood mononuclear-cell (PBMC) and CD8 CTL clones (B. Lethé, Ludwig Institute for Cancer Research [Brussels, Belgium], oral communication, 2006; and Lurquin et al20 ). For CD8β q-PCR, the upstream primer was 5′CTTCTGCATGATCGTCGGGA3′ (Eurogentec, Liège, Belgium), the downstream primer was 5′GGCCCTTCTGGGTCTCTG3′, and the probe was 5′6-FAM-CCCACCACTGCCCAGCCCAC-TAMRA3′ (Eurogentec). Annealing temperature was 60°C. For CCR7 q-PCR, the upstream primer was 5′CGTGCTGGTGGTGGCTCT3′, the downstream primer was 5′TGCCCAGTAGGCCCACGAA3′, and the Taqman probe was 5′6-FAM-CATCGGAGACAACACCACAGTGGACTA-TAMRA3′. Annealing temperature was 60°C. For CD45RA q-PCR, upstream primer was 5′ACAGCAAAGATGCCCAGT3′ and matched with exon 4 that is spliced in the CD45RO isoform. The downstream primer was 5′TGTGGTTGAAATGACAGCG3′ and matched with exon 7. The probe was 5′6-FAM-ACCTACTCACACCACTGCATTCT-Darquencher3′ matching with exon 4. Annealing temperature was 57°C.
Ex vivo analysis of CD8+ blood cells
Peripheral-blood mononuclear cells were isolated from standard buffy coat preparations obtained from hemochromatosis donors by Lymphoprep (Axix-Shiel PoCAS, Oslo, Norway). CD8+ T cells were then isolated by positive selection with anti-CD8–coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the magnetic selection was up to 97%. Selected CD8+ cells were labeled with an FITC-conjugated anti-CCR7 antibody and a PE-Cy5–conjugated anti-CD45RA antibody. Sorted cells were labeled with the PE-fluorescent dye PKh26 (Sigma, St Louis, MO) at 2 μM. About 3 × 105 cells were stimulated with 5 × 105 anti-CD3/anti-CD28–coated beads (Dynal) in 2 mL Iscove medium supplemented with AAGβ, 1% of heat inactivated autologous human serum, and IL-2 (2 U/mL).
Results
Lack of relation between CD45RA expression and functional phenotype of CTL clones
Melanoma patients have been vaccinated with autologous mature dendritic cells pulsed with MAGE-3 peptide EVDPIGHLY, which is presented by HLA-A1 molecules.18 We examined a series of anti–MAGE-3.A1 CTL clones that had been obtained from independent microcultures of blood cells after 3 to 5 antigenic stimulations performed at approximately 2-week intervals.17 The clones were selected on the basis of their ability to be stained with HLA-A1/MAGE-3 complexes, hereafter referred to as A1/MAGE-3 tetramer. They were also selected for their ability to lyse both HLA-A1 cells pulsed with the peptide and HLA-A1 tumor cells expressing MAGE-3.
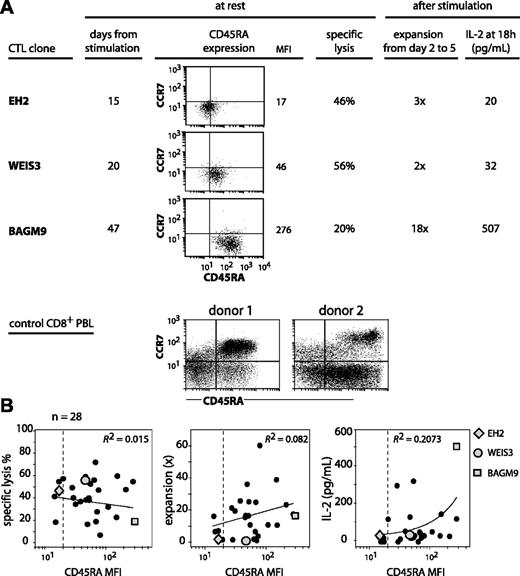
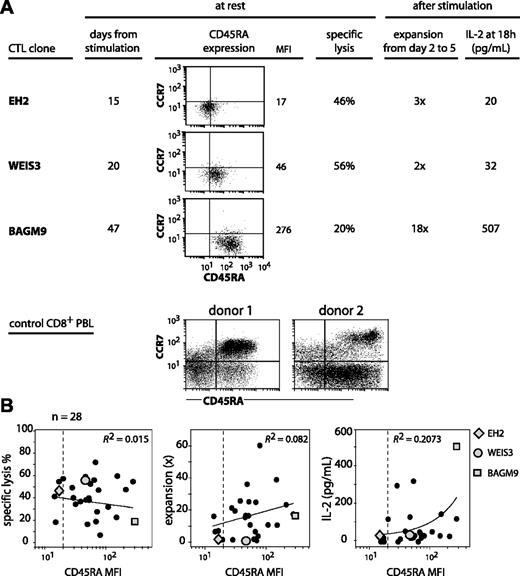
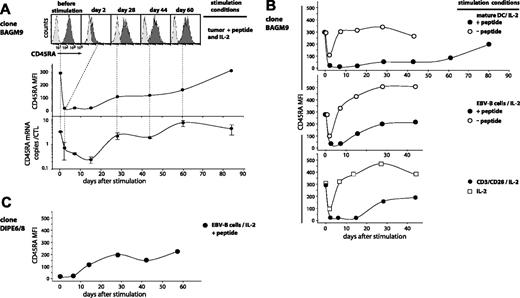
We set out to analyze CTL clones for CD45RA and CCR7 expression to examine how they related to the subgroups defined by the linear differentiation model on blood CD8 T cells.8,10 The anti–MAGE-3.A1 CTL clones that were analyzed had been stimulated with their antigen 2 to 7 weeks before. All had a CCR7– phenotype (Figure 1A). In contrast, their CD45RA surface expression was variable with a mean fluorescence intensity (MFI) spread over a 20-fold range. On some clones, the CD45RA surface expression was as low as on the CD45RA–CCR7– subset of CD8+ PBMCs, and on others it was as high as the CD45RA+CCR7– subset, as shown in Figure 1A for 3 representative CTL clones. The clones were evaluated for their lytic activity on specific targets. Their ability to proliferate and to produce IL-2 upon antigenic stimulation was also evaluated. Contrary to common assertions regarding the terminally differentiated state of CD45RA+CCR7– CD8+ T cells, we found no positive or negative correlation between CD45RA expression and proliferation potential. As seen in Figure 1A, CD45RAhigh clone BAGM9 proliferated much better upon antigenic stimulation and produced much more IL-2 than CD45RAlow clone EH2. In addition, clone BAGM9 had a lower lytic activity than clone EH2. The analysis of 28 CTL clones did not reveal any correlation between on the one hand their expression of CD45RA and on the other hand their lytic activity at rest and their proliferation and production of IL-2 upon restimulation (Figure 1B).
CD45RA/CCR7 phenotype and functional properties of anti–MAGE-3.A1 CTL clones. (A) The CTL clones from cancer patients were labeled in the same conditions as the CD8+ PBLs from donors. Cells were labeled for 15 minutes at room temperature with an FITC-conjugated anti-CCR7 antibody, a PE-conjugated anti-CD45RA antibody, and an APC-conjugated anti-CD8 antibody. Only the living CD8+ PBMCs are shown. Quadrant limits were positioned so as to include in the lower left quadrant 99% of the cells labeled with a control isotype-matched antibody. Lysis was tested by flow cytometry with CTL clones that had been stimulated 15 to 47 days before. The targets were EBV-B cells pulsed with the MAGE-3 peptide. Target cells were mixed with the CTL at an effector-target ratio of 3 and the number of living CD19+ target cells was estimated after a 3-hour coculture. Expansion and IL-2 release were estimated after antigenic stimulation. Twenty thousand CTLs were cocultured with 20 000 HLA-A1 EBV-B cells pulsed with the MAGE-3 peptide. Considering that up to 75% of the CTLs died within 2 days, the expansion of the clones was evaluated in a flow cytometer by counting the number of CD8+ cells at day 2 and at day 5. The presence of IL-2 was evaluated in the supernatant of the coculture using cytometric bead arrays. (B) A total of 28 anti–MAGE-3.A1 CTL clones was tested in the conditions described in panel A. Ninety-nine percent of the cells labeled with the control isotype-matched antibody had a fluorescence lower than the limit indicated by the dashed line. The best correlation curve and the coefficient of determination (R2) are indicated.
CD45RA/CCR7 phenotype and functional properties of anti–MAGE-3.A1 CTL clones. (A) The CTL clones from cancer patients were labeled in the same conditions as the CD8+ PBLs from donors. Cells were labeled for 15 minutes at room temperature with an FITC-conjugated anti-CCR7 antibody, a PE-conjugated anti-CD45RA antibody, and an APC-conjugated anti-CD8 antibody. Only the living CD8+ PBMCs are shown. Quadrant limits were positioned so as to include in the lower left quadrant 99% of the cells labeled with a control isotype-matched antibody. Lysis was tested by flow cytometry with CTL clones that had been stimulated 15 to 47 days before. The targets were EBV-B cells pulsed with the MAGE-3 peptide. Target cells were mixed with the CTL at an effector-target ratio of 3 and the number of living CD19+ target cells was estimated after a 3-hour coculture. Expansion and IL-2 release were estimated after antigenic stimulation. Twenty thousand CTLs were cocultured with 20 000 HLA-A1 EBV-B cells pulsed with the MAGE-3 peptide. Considering that up to 75% of the CTLs died within 2 days, the expansion of the clones was evaluated in a flow cytometer by counting the number of CD8+ cells at day 2 and at day 5. The presence of IL-2 was evaluated in the supernatant of the coculture using cytometric bead arrays. (B) A total of 28 anti–MAGE-3.A1 CTL clones was tested in the conditions described in panel A. Ninety-nine percent of the cells labeled with the control isotype-matched antibody had a fluorescence lower than the limit indicated by the dashed line. The best correlation curve and the coefficient of determination (R2) are indicated.
Time course of CD45RA expression after stimulation
We observed that a higher expression of CD45RA was usually found on those clones that had been left without antigenic stimulation for a long time. This suggested that CD45RA expression might increase with the time elapsed in the absence of antigenic stimulation. We therefore evaluated CD45RA expression at various times after stimulation.
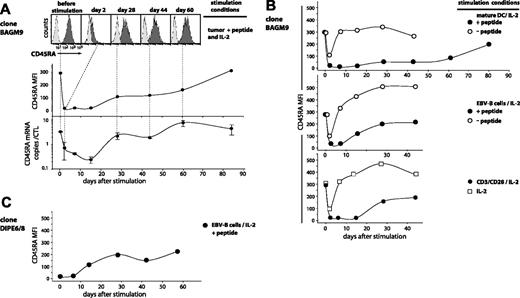
CD45RAhigh CTL clone BAGM9, which had not been in contact with antigen for 47 days, was stimulated with tumor cells pulsed with peptide in the presence of IL-2. At various times after stimulation, the surface expression of CD45RA was measured by flow cytometry. A homogeneous decrease in surface expression by approximately 14-fold was observed on day 2 after stimulation. As a result, 60% of the cells had a fluorescence lower than the upper 1% of the isotype control, defining them as CD45RA– (Figure 2A). Surface expression remained very low until day 15. We also evaluated by quantitative PCR the amount of the CD45RA final transcript, which results from a splicing that differs from that of CD45RO. The number of CD45RA transcripts also decreased, being 5-fold lower on day 2 and 10-fold lower on day 15 (Figure 2A). It is noteworthy that surface expression dropped more rapidly than the number of transcripts, possibly because of endocytic removal of CD45RA after antigenic stimulation. After day 15, both the transcripts and the surface expression of CD45RA increased gradually. By day 84, they had regained their initial level. All the CD45RA+ CTL clones that were tested showed CD45RA decrease after antigenic stimulation, but the importance of the decrease and the kinetics of the re-expression were variable from one clone to another. As expected, the transcription and surface expression of CD45RO isoform varied inversely relative to CD45RA (data not shown).
The contact of CTLs with mature dendritic cells pulsed with the peptide produced a longer period of low CD45RA surface expression than pulsed EBV-B cells, tumor cells, or anti-CD3/CD28 antibodies, consistent with the idea that they induce optimal stimulation of T cells (Figure 2B). Adding to CTL BAGM9 dendritic cells or EBV-B cells in the absence of the antigen, adding cytokines only, or simply mixing the CTLs generated a short-lived 3-fold decrease of CD45RA surface expression (Figure 2B). But we did not observe this decrease with another CTL clone.
In addition to MAGE-3–specific CTLs, we also tested HLA-A2–restricted CTL clone DIPE6/8 directed against an EBV antigen encoded by gene BMLF1. On the day on which this CTL was stimulated with peptide-pulsed cells, it was already CD45RA– because of a recent stimulation with anti-CD3/CD28 antibodies (Figure 2C). Ten days after stimulation with the antigenic peptide, the expression of CD45RA gradually increased and reached after one month a plateau level of CD45RA that was comparable with that observed with the anti–MAGE-3 CTL clone.
Time course of CD45RA expression on antigen-stimulated CTLs. (A) BAGM9 CTLs (2 × 105), which had a CD27–CD28– phenotype, were stimulated with 2 × 105 MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide, in the presence of IL-2. Half of the medium was replaced at 2-week intervals by fresh medium and IL-2. Samples were collected from the coculture at different time points. Cells were labeled with an APC-conjugated anti-CD8 antibody and either a PE-conjugated anti-CD45RA antibody (black histograms) or a control isotype-matched antibody (gray histograms). Only the living CD8+ cells are shown in the figure. Surface expression of CD45RA is indicated as the MFI after subtraction of the MFI of the cells labeled with the control isotype-matched antibody. The number of CD45RA transcripts was evaluated by quantitative PCR, and the results were normalized to the CD8 transcripts. (B) Clone BAGM9 was stimulated with beads coated either with anti-CD3 and anti-CD28 antibodies, mature dendritic cells, or EBV-B cells. When indicated, cells were pulsed with the MAGE-3 peptide. Samples were collected from the cocultures at different time points and the surface expression of CD45RA was evaluated as described in panel A. (C) DIPE6/8 CTLs (3 × 105) were stimulated with 1.5 × 105 HLA-A2 EBV-B cells pulsed with the BMLF1 peptide, in the presence of IL-2. Cells were cultured, sampled, and analyzed in the same conditions as in panels A-B.
Time course of CD45RA expression on antigen-stimulated CTLs. (A) BAGM9 CTLs (2 × 105), which had a CD27–CD28– phenotype, were stimulated with 2 × 105 MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide, in the presence of IL-2. Half of the medium was replaced at 2-week intervals by fresh medium and IL-2. Samples were collected from the coculture at different time points. Cells were labeled with an APC-conjugated anti-CD8 antibody and either a PE-conjugated anti-CD45RA antibody (black histograms) or a control isotype-matched antibody (gray histograms). Only the living CD8+ cells are shown in the figure. Surface expression of CD45RA is indicated as the MFI after subtraction of the MFI of the cells labeled with the control isotype-matched antibody. The number of CD45RA transcripts was evaluated by quantitative PCR, and the results were normalized to the CD8 transcripts. (B) Clone BAGM9 was stimulated with beads coated either with anti-CD3 and anti-CD28 antibodies, mature dendritic cells, or EBV-B cells. When indicated, cells were pulsed with the MAGE-3 peptide. Samples were collected from the cocultures at different time points and the surface expression of CD45RA was evaluated as described in panel A. (C) DIPE6/8 CTLs (3 × 105) were stimulated with 1.5 × 105 HLA-A2 EBV-B cells pulsed with the BMLF1 peptide, in the presence of IL-2. Cells were cultured, sampled, and analyzed in the same conditions as in panels A-B.
Time course of CCR7 expression on CTL clones after antigenic stimulation
Several groups have reported that CD45RA–CCR7– CD4+ and CD8+ blood T cells express CCR7 after TCR stimulation with anti-CD3 and anti-CD28 antibodies.1,12,16 We examined this process in CTL clones that had not been stimulated for at least 14 days.
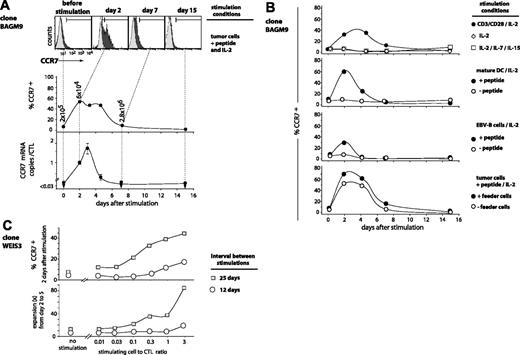
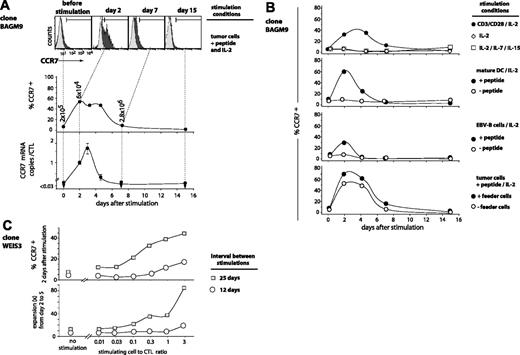
Clone BAGM9 was stimulated with tumor cells pulsed with peptide. Two days after antigenic stimulation, 52% of the cells showed a significant CCR7 surface expression (Figure 3A). It remained high until day 5 and returned to basal level by day 7. The CCR7 transcripts, which were undetectable at rest, also increased rapidly after stimulation. The number of CCR7 mRNAs reached a maximum at day 3 and then decreased rapidly to become undetectable at day 7.
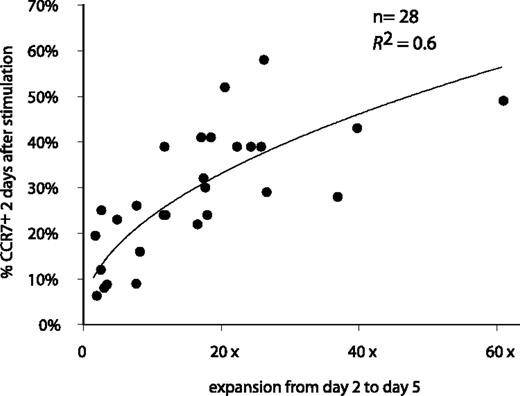
A similar cyclic variation of the CCR7 expression after antigenic stimulation was observed for all 28 analyzed CTL clones, but the percentage of cells that switched on CCR7 varied from one clone to another, ranging from 5% to 58% of the cells (Figure 4). Invariably, CCR7 surface expression disappeared within 2 weeks.
TCR stimulation, provided either by the antigen or by CD3/CD28 ligation, was strictly required for the up-regulation of CCR7 (Figure 3B). IL-2 alone did not trigger expression of CCR7. This also applied to the combination of IL-2, IL-7, and IL-15, which is known to be sufficient for the induction of proliferation and production of IFN-γ, TNF-β, perforin, and granzyme B.21 The highest percentages of cells switching on CCR7 were obtained after stimulation with peptide-pulsed mature dendritic cells or peptide-pulsed tumor cells together with EBV-B feeder cells. A longer resting period before stimulation resulted in a higher percentage of cells switching on CCR7, and in a more intense proliferation (Figure 3C). The percentage was also higher when the number of presenting cells per CTL was higher (Figure 3C). Among all the stimulation conditions that were tested, none induced a CCR7 expression on all the cells, even though activation marker CD25 was up-regulated in more than 99% of the CTLs (data not shown).
Time course of CCR7 expression on antigen-stimulated CTLs. (A) BAGM9 CTLs (2 × 105) were stimulated with 2 × 105 MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide in medium containing IL-2 (50 U/mL). Half of the medium was replaced at 2-week intervals by fresh medium and IL-2. Samples were collected from the coculture at different time points. Cells were labeled with an APC-conjugated anti-CD8 antibody and either an FITC-conjugated anti-CCR7 antibody (black histograms) or a control isotype-matched antibody (gray histograms). Only the living CD8+ cells are shown. Surface expression of CCR7 is indicated as the percentage of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody. The number of CCR7 transcripts was evaluated by quantitative PCR, and the results were normalized to the CD8 transcripts. (B) Clone BAGM9 was stimulated with either beads coated with anti-CD3 and anti-CD28 antibodies, mature dendritic cells, or EBV-B cells. If indicated, cells were pulsed with the MAGE-3 peptide. Samples were collected from the cocultures at different time points and the CCR7 surface expression was evaluated. (C) CTL clone WEIS3 had a CD27–CD28– phenotype. Some CTLs were stimulated with their antigen 25 days before and some others 12 days before. CTLs (105) were stimulated with MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide at the indicated stimulating-cell/CTL ratio. The surface expression of CCR7 was measured 2 days after stimulation. Cells were labeled with an FITC-conjugated anti-CCR7 antibody. The percentage of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody is indicated on the y-axis. The expansion of the clones was evaluated in a flow cytometer by counting the number of CD8+ cells at day 2 and at day 5.
Time course of CCR7 expression on antigen-stimulated CTLs. (A) BAGM9 CTLs (2 × 105) were stimulated with 2 × 105 MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide in medium containing IL-2 (50 U/mL). Half of the medium was replaced at 2-week intervals by fresh medium and IL-2. Samples were collected from the coculture at different time points. Cells were labeled with an APC-conjugated anti-CD8 antibody and either an FITC-conjugated anti-CCR7 antibody (black histograms) or a control isotype-matched antibody (gray histograms). Only the living CD8+ cells are shown. Surface expression of CCR7 is indicated as the percentage of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody. The number of CCR7 transcripts was evaluated by quantitative PCR, and the results were normalized to the CD8 transcripts. (B) Clone BAGM9 was stimulated with either beads coated with anti-CD3 and anti-CD28 antibodies, mature dendritic cells, or EBV-B cells. If indicated, cells were pulsed with the MAGE-3 peptide. Samples were collected from the cocultures at different time points and the CCR7 surface expression was evaluated. (C) CTL clone WEIS3 had a CD27–CD28– phenotype. Some CTLs were stimulated with their antigen 25 days before and some others 12 days before. CTLs (105) were stimulated with MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide at the indicated stimulating-cell/CTL ratio. The surface expression of CCR7 was measured 2 days after stimulation. Cells were labeled with an FITC-conjugated anti-CCR7 antibody. The percentage of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody is indicated on the y-axis. The expansion of the clones was evaluated in a flow cytometer by counting the number of CD8+ cells at day 2 and at day 5.
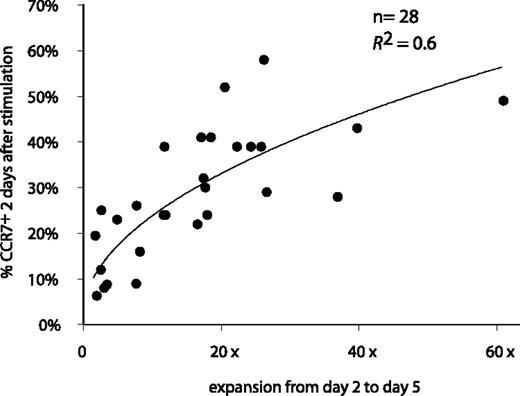
Correlation between the percentage of CCR7+ cells after stimulation and clone expansion. The 28 anti–MAGE-3.A1 clones were stimulated with MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide and IL-2. Samples were collected 48 hours after stimulation. Cells were labeled with an APC-conjugated anti-CD8 antibody and either an FITC-conjugated anti-CCR7 antibody or a control isotype-matched antibody. Surface expression of CCR7 is expressed as the percentage of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody. Expansion was estimated by counting the number of CD8+ cells at days 2 and 5 by flow cytometry. The best correlation curve and the coefficient of determination (R2) are indicated.
Correlation between the percentage of CCR7+ cells after stimulation and clone expansion. The 28 anti–MAGE-3.A1 clones were stimulated with MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide and IL-2. Samples were collected 48 hours after stimulation. Cells were labeled with an APC-conjugated anti-CD8 antibody and either an FITC-conjugated anti-CCR7 antibody or a control isotype-matched antibody. Surface expression of CCR7 is expressed as the percentage of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody. Expansion was estimated by counting the number of CD8+ cells at days 2 and 5 by flow cytometry. The best correlation curve and the coefficient of determination (R2) are indicated.
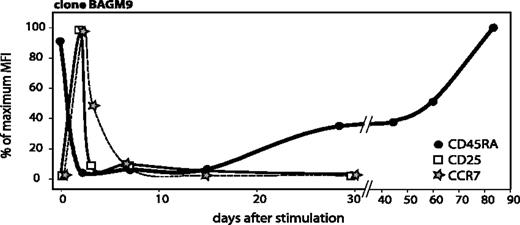
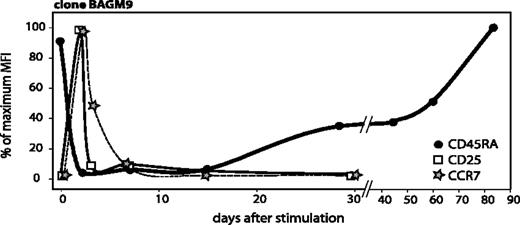
Upon stimulation, CD25 and CCR7 reappeared very rapidly, reaching a maximum around day 2 (Figure 5). CD25 disappeared by day 3 and CCR7 by day 7. We would like to call activated T cells those T cells that have been stimulated by their antigen fewer than 10 ± 3 days before, as during that period they proliferate significantly, and they transiently express CD25 and CCR7. The gradual process of re-expression of CD45RA begins after the disappearance of CD25 and CCR7, provided that T cells do not receive further antigenic stimulation (Figure 5).
Time course of CD45RA, CCR7, and CD25 surface expression after antigenic stimulation. The surface expressions of CD45RA and CCR7 were already shown in Figures 2A and 3A. CD25 surface expression was evaluated on samples collected from the cocultures at different time points. Cells were labeled with an APC-conjugated anti-CD8 antibody and either a PE-conjugated anti-CD25 antibody or a control isotype-matched antibody. Surface expression of these 3 markers is indicated as a percentage of the highest MFI, which was 318 for CD45RA, 28 for CCR7, and 789 for CD25.
Time course of CD45RA, CCR7, and CD25 surface expression after antigenic stimulation. The surface expressions of CD45RA and CCR7 were already shown in Figures 2A and 3A. CD25 surface expression was evaluated on samples collected from the cocultures at different time points. Cells were labeled with an APC-conjugated anti-CD8 antibody and either a PE-conjugated anti-CD25 antibody or a control isotype-matched antibody. Surface expression of these 3 markers is indicated as a percentage of the highest MFI, which was 318 for CD45RA, 28 for CCR7, and 789 for CD25.
We observed a correlation between the percentage of cells that switched on CCR7 and the degree of clone expansion following antigenic stimulation, suggesting that cells that up-regulated CCR7 multiplied more (Figure 4). In contrast, the production of TNF-α, IFN-γ, IL-4, IL-10, or IL-2 upon stimulation was not correlated with CCR7 expression (data not shown).
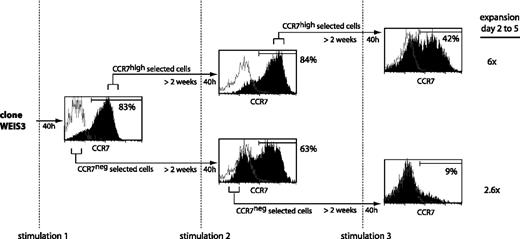
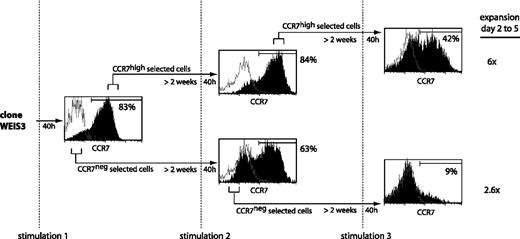
We examined whether the subset of T cells that up-regulated CCR7 following one stimulation had a higher tendency than the others to up-regulate CCR7 following subsequent stimulations. To this end, we tried to separate the CCR7high and the CCR7low subsets observed following stimulation of CTL clone WEIS3. After 40 hours, 83% of the cells expressed CCR7 (Figure 6). The CCR7high and CCR7neg subsets were selected in a flow cytometer. Two weeks later, both subpopulations were stimulated again. Both sets produced CCR7+ and CCR7– cells in proportions that were similar to those observed after the first stimulation. However, upon an additional sorting of the CCR7high and CCR7neg subsets, followed by a stimulation 2 weeks later, the CCR7high subset produced 42% of CCR7+ cells, whereas the CCR7neg subset produced only 9% CCR7+ cells (Figure 6). The CCR7high and the CCR7neg selected subsets showed expansion of 6-fold and 2.6-fold, respectively, in line with the observations described in Figure 4. The same result was obtained in 3 independent experiments, suggesting that it may be possible to select T cells that have lastingly lost their potential to up-regulate CCR7. Our observation of a higher proliferation of the cells that up-regulated CCR7 is in accordance with observations made with polyclonal blood cells.16 We observed no difference between the 2 subsets in terms of surface markers CD27 and CD28, which were both negative.
Ex vivo analysis of CD8+ blood cells
In the experiment reported by Champagne et al,10 unfractionated blood cells labeled with cell-division marker CFSE were stimulated with anti-CD3/CD28 antibodies. Taking samples after time intervals ranging from 36 to 60 hours, they observed no dilution of CFSE among the CD8+ cells that were CD45RA+ and CCR7– at that time. They concluded that CD45RA+CCR7– cells had no proliferation potential in keeping with the notion of terminally differentiated T cells.
Selection of a subpopulation having lost the ability to switch on CCR7 upon stimulation. CTL clone WEIS3, which had a CD27–CD28– phenotype, was stimulated with MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide and IL-2. Cells were stained 40 hours after each stimulation with an anti-CCR7 antibody, with a biotinylated anti–mouse IgG2a antibody, and with a PE-Cy5–conjugated streptavidin (black histograms). Control cells were stained with an isotype-matched antibody instead of the anti-CCR7 antibody (empty histograms). Cells with the highest fluorescence (CCR7high) and cells with the lowest fluorescence (CCR7neg) were selected by flow cytometry, cultured with IL-2, and restimulated approximately 2 weeks later. The percentages indicated in the figure correspond to the fraction of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody. After 2 rounds of selection and a further stimulation, 60% of the cells were surviving at day 2 for both subpopulations. Expansion was estimated at days 2 and 5 after stimulation by counting the CD8+ cells by flow cytometry.
Selection of a subpopulation having lost the ability to switch on CCR7 upon stimulation. CTL clone WEIS3, which had a CD27–CD28– phenotype, was stimulated with MZ2-MEL.43 tumor cells pulsed with the MAGE-3 peptide and IL-2. Cells were stained 40 hours after each stimulation with an anti-CCR7 antibody, with a biotinylated anti–mouse IgG2a antibody, and with a PE-Cy5–conjugated streptavidin (black histograms). Control cells were stained with an isotype-matched antibody instead of the anti-CCR7 antibody (empty histograms). Cells with the highest fluorescence (CCR7high) and cells with the lowest fluorescence (CCR7neg) were selected by flow cytometry, cultured with IL-2, and restimulated approximately 2 weeks later. The percentages indicated in the figure correspond to the fraction of the cells having a fluorescence intensity above 99% of the cells labeled with the control isotype-matched antibody. After 2 rounds of selection and a further stimulation, 60% of the cells were surviving at day 2 for both subpopulations. Expansion was estimated at days 2 and 5 after stimulation by counting the CD8+ cells by flow cytometry.
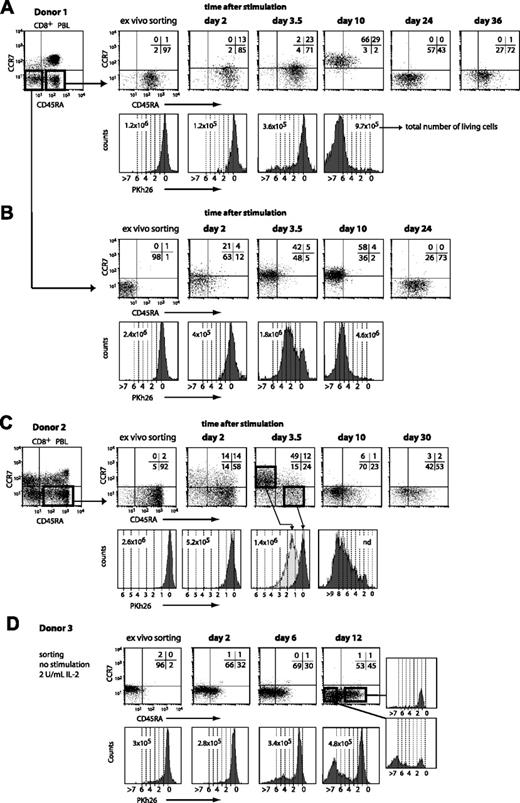
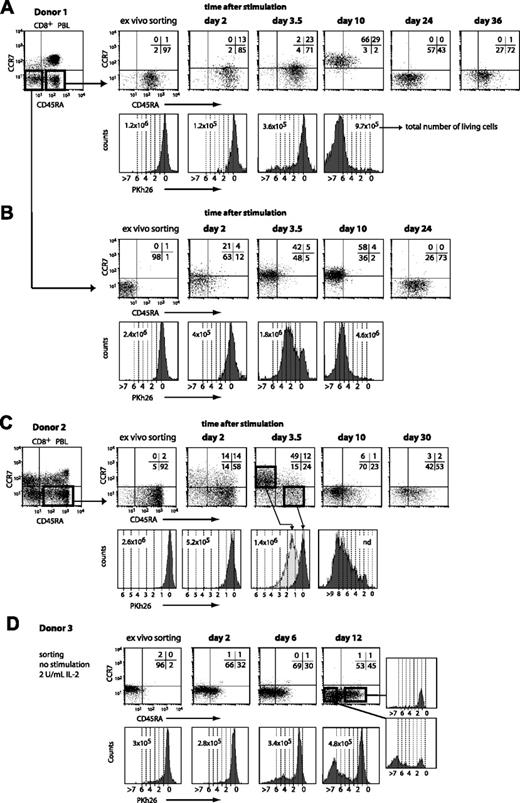
To re-examine the proliferation potential of CD45RA+CCR7– CD8+ T cells, we designed our experiment differently. After selecting CD8+ blood cells by magnetic sorting, CD45RA+CCR7– cells were sorted by flow cytometry and they were labeled with the PKh26 dye to follow cell division. The cells were then stimulated with beads coated with anti-CD3/CD28 antibodies. The cells were examined at various times thereafter to assess CCR7 and CD45RA surface expression and Pkh26 dilution. We tested blood from various donors and we observed the same evolutions in all the experiments, but the time courses varied considerably. In the first experiment shown in Figure 7A, by day 3.5 most of the cells had remained CD45RA+CCR7– and had not divided. But by day 10, all the cells had become CCR7+ and most had lost CD45RA. Most of these cells had undergone from 5 to more than 7 divisions. By day 24, all the cells had lost CCR7 and the proportion of CD45RA+ cells had increased. By day 36, most of the cells were again CD45RA+CCR7–. An experiment with CD45RA+CCR7– cells from another donor is shown in Figure 7C. Here the evolution showed a faster time course. At day 2, 28% of the cells were CCR7+, half of those being also CD45RA–. At day 3.5, 50% of the cells were in a CD45RA–CCR7+ cluster and had undergone 1 to 3 divisions. In contrast, the 24% of the cells that had remained CD45RA+CCR7– had not divided at all. By day 10, all the cells were again CCR7–, most being CD45RA–. Most of the cells had divided 4 to 9 times. By day 30, half the cells were CD45RA+CCR7–. These results indicate clearly that the CD45RA+CCR7– compartment contains many cells that have a considerable proliferative capacity upon stimulation. Champagne et al did not observe this proliferation because their experimental design precluded observation of the CD45RA+CCR7– cells that became CD45RA– or CCR7+ following stimulation.10
In addition to the CD45RA+CCR7– cells, we also sorted CD45RA–CCR7– cells from the blood CD8+ T cells of donor 1 (Figure 7B). The cells were also labeled with the PKh26 dye and were stimulated with beads coated with anti-CD3/CD28 antibodies. By day 3.5, half of the cells had acquired CCR7 and a majority had made between 1 and 4 divisions. By day 24, all the cells had lost CCR7 and 73% had acquired CD45RA expression. We conclude that a long period without antigenic stimulation results in the re-expression of CD45RA on most CD45RA–CCR7– CD8+ T cells.
CD45RA and CCR7 expression and proliferation of different CD8+ blood-cell subsets upon stimulation. Blood CD8+ cells were positively selected by magnetic sorting and labeled with an FITC-conjugated anti-CCR7 antibody and a PE-Cy5–conjugated anti-CD45RA antibody. The CD45RA+CCR7– and CD45RA–CCR7– subsets were sorted by flow cytometry, labeled with PKh26, and distributed at approximately 3 × 105 cells per well. Cells were either stimulated with beads coated with anti-CD3 and anti-CD28 antibodies in the presence of 2 U/mL IL-2 (A-C) or kept in culture with 2 U/mL IL-2 (D). Samples were collected at different time points after stimulation, and CD45RA/CCR7 surface expression was evaluated with the antibodies used for the sorting. Quadrant limits were positioned so as to include in the lower left quadrant 99% of the nonlabeled cells, and the numbers correspond to the percentage of cells in each quadrant. Proliferation was measured by the dilution of the Pkh26 dye and the dashed lines correspond to the number of divisions.
CD45RA and CCR7 expression and proliferation of different CD8+ blood-cell subsets upon stimulation. Blood CD8+ cells were positively selected by magnetic sorting and labeled with an FITC-conjugated anti-CCR7 antibody and a PE-Cy5–conjugated anti-CD45RA antibody. The CD45RA+CCR7– and CD45RA–CCR7– subsets were sorted by flow cytometry, labeled with PKh26, and distributed at approximately 3 × 105 cells per well. Cells were either stimulated with beads coated with anti-CD3 and anti-CD28 antibodies in the presence of 2 U/mL IL-2 (A-C) or kept in culture with 2 U/mL IL-2 (D). Samples were collected at different time points after stimulation, and CD45RA/CCR7 surface expression was evaluated with the antibodies used for the sorting. Quadrant limits were positioned so as to include in the lower left quadrant 99% of the nonlabeled cells, and the numbers correspond to the percentage of cells in each quadrant. Proliferation was measured by the dilution of the Pkh26 dye and the dashed lines correspond to the number of divisions.
To analyze the evolution of CD45RA–CCR7– cells in the absence of stimulation, such cells were sorted from the blood CD8+ T cells of another donor (Figure 7D). The cells were labeled with the PKh26 dye and kept in culture with only 2 U/mL IL-2, but were not stimulated with antibody-coated beads. By day 12, 45% of the cells had become CD45RA+. These cells had made only one division. Fifty-three percent of the cells had remained CD45RA–. The majority of these cells had made more than 5 divisions, suggesting that they may be derived from a small subset of the original population. Considering that 8% of the CD45RA–CCR7– cells from donor 3 expressed CD25 (Table 1), suggesting a recent activation event, it is possible that these cells could account for the proliferating cells that had remained CD45RA– at day 12.
Returning to the observations that upon stimulation most cells of the CD45RA+CCR7– subset became CCR7+ and proliferated (Figure 7A,C), one could have argued that the CD45RA+ cells die, whereas a small contamination of CD45RA– cells proliferate heavily to produce the CCR7+ cells. These CD45RA– cells could have contributed many of the 10% to 20% of the cells that were alive 2 days after stimulation. However, the percentage of surviving cells was similar in the experiments performed with the CD45RA–CCR7– cells of patient 1 and their degree of proliferation was also similar (Figure 7B). We therefore conclude that most of the cells of the CD45RA+CCR7– subset that became CCR7+ and proliferated were indeed CD45RA+CCR7– cells. A low proportion of surviving cells was also observed on day 2 after stimulation with the CTL clones. Only 30% of the BAGM9 CTLs survived after stimulation with peptide-pulsed tumor cells (Figure 3), and only 13% after stimulation with anti-CD3/CD28 antibodies (data not shown).
If the CD45RA+CCR7– blood CD8+ T cells are cells that have not seen their antigen for several weeks, this subset of cells should not contain recently activated T cells. To estimate the proportion of activated T cells, we sorted blood CD8+ T cells of various donors by magnetic sorting and immediately labeled them with antibodies against activation marker CD25 (Table 1). For all the donors, the lowest percentage of CD25+ cells, 1% or less, was observed in the CD45RA+CCR7– subset. The CD45RA–CCR7+ compartment contained the highest proportion of CD25+ cells, as expected if most activated T cells express CCR7 and if for primary blood cells the peak of CD25 expression is shorter than the CCR7 peak, as observed for CTL clones (Figure 5). The presence of some CD25+ cells among the CD45RA–CCR7– cells could reflect the fact that upon activation only a fraction of the T cells re-express CCR7, as observed with the CTL clones.
Discussion
The linear differentiation model proposes that upon antigenic stimulation the naive CD8 T cells, which are CD45RA+CCR7+, lose CD45RA to become central memory T cells, then lose CCR7 to become effector memory cells. The observation that CCR7 can transiently be re-expressed by CCR7– memory CD8+ T cells in the days following antigenic stimulation, as shown in previous reports8,15,16 and confirmed here, implies that the stages proposed by the linear differentiation model define only resting T cells.
Some CCR7– CD8+ T cells express CD45RAand these cells, which have been named TEMRA, have been considered to be terminally differentiated because of their high content of perforin, because of their ability to secrete IFN-γ but not IL-2 following stimulation, and also because of their very low proliferation following TCR engagement.9,10 But this subset of blood T cells has been observed to be heterogeneous in terms of expression of granzyme B, FasL, TNF-α, and IFN-γ.11 Our analysis of resting CTL clones, which are always CCR7–, did not reveal any correlation between, on the one hand, their level of CD45RA expression and, on the other hand, their ability to secrete IL-2 and their lytic activity. CTL clones that were completely CD45RA+ showed considerable proliferative potential following antigenic stimulation. We extended these observations to blood cells, and observed that, in accordance with previous reports on antivirus T cells,12,13 the CD8+ CD45RA+CCR7– subset showed also considerable proliferation following TCR engagement. We conclude that CD45RA+CCR7– CD8+ T cells are by no means terminally differentiated.
We observed that, when cells of permanent CD8+ CTL clones, which were CD45RA+, received a TCR stimulation, CD45RA was lost at the onset of the activation phase, which lasted for about 2 weeks. The activation phase was followed by a longer phase of re-expression of CD45RA that appeared to be completed on all the cells after about 8 weeks. Thus the level of CD45RA increased with the time elapsed since the last antigenic stimulation. For CD45RA+CCR7– blood CD8+ T cells, which also lose CD45RA after stimulation in vitro, re-expression of CD45RAwas observed on a majority of cells after about 5 weeks. For CD45RA–CCR7– blood CD8+ T cells, we observed the appearance of CD45RA on a large majority of cells 4 weeks after stimulation. We also observed the appearance of CD45RA on nearly half of the CD45RA–CCR7– blood CD8+ T cells kept in culture without stimulation for 2 weeks. We conclude that CD45RA– and CD45RA+ CCR7– CD8+ blood T cells have the potential to acquire or reacquire CD45RA expression following a period without antigenic stimulation, but we do not claim that all CD45RA– CCR7– CD8+ T cells have this potential.
The reacquisition of CD45RA following a long absence of antigen does not appear to apply to CCR7– CD4+ memory cells. In the blood, very few CD45RA+CCR7– CD4+ cells are observed.8 We have tested one anti–MAGE-3 CD4+ T-cell clone up to 90 days after an antigenic stimulation and have not observed acquisition of CD45RA.
A previous report showed that CCR7-positive CD45RA– CD8+ T cells evolved into CD45RA+CCR7– CD8+ T cells in the presence of IL-7 and IL-15 when no antigenic stimulation was provided.9,14,22 Our results suggest that the crucial factor was the absence of antigenic stimulation, but we agree that the presence of IL-7 and IL-15 may speed up the acquisition of CD45RA.9,22
We therefore would like to call “activated T cells” those CD8+ T cells that have been stimulated by their antigen fewer than 10 ± 3 days before, as during that period they proliferate significantly, all of them transiently express CD25, and some of them express CCR7. After that time, only the lymphocytes that do not receive further antigenic stimulation enter into a slow and gradual process of re-expression of CD45RA, which is completed at the latest 10 weeks after stimulation, and we propose to name the lymphocytes that are in this process of reacquiring CD45RA “transitional resting T cells.” We propose to name “stable resting T cells” the lymphocytes that have reached the plateau for the expression of CD45RA.
The view that CD45RA is not a marker of terminal differentiation of memory CD8+ T cells, but results from a long-lasting absence of antigen, provides an alternative explanation for several published observations regarding the CD45RA phenotype of CTLs found in the course of various viral infections of humans. During the primary phase of infectious mononucleosis when both lytic and latent EBV proteins are expressed, the CTLs directed against these antigens were observed to have a uniform CD45RA– phenotype.23 In the persistent carrier state, latent EBV proteins are expressed continuously in infected cells, while the lytic proteins are found only in the very rare cells that are in lytic cycle.24 At that stage, almost all of the CTLs directed against the latent proteins were found to be CD45RA–, whereas most of those directed against the lytic proteins were CD45RA+.22,23,25 This could be readily explained in terms of lack of antigenic stimulation allowing evolution into the CD45RA+ phenotype.
In an interesting study performed on patients in chronic phase of HIV infection, it was observed that the anti-HIV CTLs were CD45RA–, whereas the anti-CMV CTLs resulting from a past infection were CD45RA+.10 The proposed interpretation was that, following the CMV infection, CTLs had been able to differentiate properly into highly effective TEMRA, whereas HIV was somehow blocking this terminal differentiation—hence a less effective response against HIV than against CMV.10,26 We agree that a virus such as HIV could impair T-cell differentiation, but our observations suggest that an alternative interpretation should be considered, namely that the relatively high viral load observed in chronic HIV infection provided continuous antigenic stimulation and that the resting period was therefore too short to allow re-expression of CD45RA. In contrast, the anti-CMV CTLs of these patients had not been restimulated for a long time, allowing CD45RA re-expression. In line with this interpretation, another report showed that the anti-CMV CTLs were CD45RA– during acute CMV infection.6
The notion that CD45RA+CD8+ T lymphocytes have seen their antigen at a distant time can also lead to reinterpretation of some observations made on cancer patients. Lymph nodes invaded with tumor cells were observed to contain a much higher proportion of CD45RA–CCR7– lymphocytes than uninvaded lymph nodes.27 As we recently found that in invaded lymph nodes and skin metastases of melanoma patients the majority of T cells appear to be directed against antigens borne by the tumor, the first observation can be readily explained: the CD45RA–CCR7–CD8+ T cells in invaded lymph nodes are stimulated by their antigen.20 In another report, the absence of CD45RA+CCR7– T cells in ascites from cancer patients was taken as evidence that the T cells could not attack the tumor cells properly as they were not fully differentiated effectors.28 We interpret this as showing that the T cells present in the ascites were stimulated by their antigens. Of course, it remains to be explained why they do not kill the tumor cells more efficiently. Finally, reports dealing with antimelanoma vaccination with antigens melan-A and gp-100 took the presence of CD45RA+CCR7– CD8+ T cells in the blood as evidence of successful vaccination.29,30 We would be inclined to draw the opposite conclusion.
To conclude, we have observed that a large fraction of CCR7– CD8+ T memory cells re-express CD45RA when they have not been in contact with their antigen for more than one month. Even though we cannot claim that this applies to all memory CD8+ T cells, this observation leads to new interpretations of several situations encountered in viral infections and cancer.
Prepublished online as Blood First Edition Paper, July 20, 2006; DOI 10.1182/blood-2005-11-007237.
Supported by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, and by a grant from the Fédération Belge contre le Cancer (Belgium). J.C. was supported by a grant from Fonds National de la Recherche Scientifique (FNRS)–TELEVIE.
J.C. designed and performed research, and wrote the paper; D.G. performed research; A.V.P. contributed essential reagents; and T.B. and P.v.d.B. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Benoît Van den Eynde for critical reading, and Mrs Nathalie Krack and Valérie Winand for editorial assistance. We thank Bernard Lethé for the setup of quantitative PCRs. We also thank Nicolina Renkvist for experiments with CD4+ T cells. The anti-EBV clone DIPE6/8 was kindly provided by Vaios Karanikas. We appreciated very much the technical assistance of Mr André Tonon, Mrs Maria Panagiotakopoulos, and Mrs Vinh Ha Thi.