Abstract
Neisseria meningitidis is one of the leading causes of bacterial meningitis and septicemia in children. Vaccines containing the purified polysaccharide capsule from the organism, a T cell-independent antigen, have been available for decades but do not appear to provide protection in infancy or immunologic memory as measured by antibody responses. By contrast, T cell-dependent serogroup C protein-polysaccharide conjugate vaccines protect against serogroup C meningococcal disease from infancy onward and prime for immunologic memory. We compared the magnitude and kinetics of plasma cell and memory B-cell responses to a meningococcal plain polysaccharide vaccine and a serogroup C glycoconjugate vaccine in adolescents previously primed with the conjugate vaccine. Plasma cell kinetics were similar for both vaccines, though the magnitude of the response was greater for the glycoconjugate. In contrast to the glycoconjugate vaccine, the plain polysaccharide vaccine did not induce a persistent immunoglobulin G (IgG) memory B-cell response. This is the first study to directly show that serogroup C meningococcal glycoconjugate vaccines induce persistent production of memory B cells and that plain polysaccharide vaccines do not, supporting the use of the conjugate vaccine for sustained population protection. Detection of peripheral blood memory B-cell responses after vaccination may be a useful signature of successful induction of immunologic memory during novel vaccine evaluation.
Introduction
Neisseria meningitidis is one of the leading causes of bacterial meningitis and septicemia in children worldwide and a cause of more than 50 000 deaths per year.1 These bacteria are encapsulated with polysaccharides that are a major determinant of virulence. The capsular polysaccharide of serogroup C N meningitidis is the antigen used in meningococcal vaccines that protect against serogroup C strains. The protection afforded by these vaccines is determined by both the persistence of anticapsular antibodies in serum and the induction of a secondary antibody response on re-encounter with the antigen (immunologic priming). Native bacterial capsular polysaccharides are T cell-independent antigens and are thus conventionally considered unable to induce immunologic priming. Conjugation (covalent linkage) of the capsular polysaccharide to carrier proteins, such as tetanus toxoid or CRM197 (a mutant peptide related to diphtheria toxoid), results in a T cell-dependent vaccine response to the polysaccharide antigen able to induce priming.2
The relative contributions of antibody persistence and priming to long-term vaccine-induced protection have become a central issue in protein-polysaccharide conjugate vaccine immunobiology. This issue was highlighted by an analysis of serogroup C meningococcal (MenC) glycoconjugate surveillance data following the introduction of the routine use of MenC vaccine in the United Kingdom. Results of the analysis suggested that vaccine efficacy is poor more than 1 year after immunization with a 3-dose primary vaccine series in infancy.3 Other studies have observed a rapid decrease in the serologic correlate of protection—the bactericidal antibody titer4 —that appears to correspond to the decrease in vaccine efficacy over a similar time scale. However, this rapid waning in vaccine efficacy and functional antibody occurs despite evidence of the persistence of immunologic priming.5 Use of the accelerated 2-3-4-month schedule in the United Kingdom indicated that serogroup C meningococcal polysaccharide conjugates may not protect against invasive serogroup C meningococcal disease beyond 1 year of age.
A better understanding of the mechanisms of antibody persistence and immunologic priming for these vaccines is likely to lead to more rational choices of vaccine formulations and schedules to provide more sustained protection. In humans, memory B cells can be detected in the peripheral blood months to decades after immunization6,7 and are thought to provide the basis for the secondary immune response to an antigen and antibody persistence.8-10 However, for any vaccine antigen, it remains uncertain how peripheral blood memory B-cell responses relate to antibody persistence or priming.10 Although plasma cell response to polysaccharide and protein-polysaccharide antigens have been studied previously,11 the kinetics of the memory B-cell response to this class of vaccines is unknown.
We hypothesized that comparing the antigen-specific B-cell response of a glycoconjugate MenC vaccine (MenCC) and a plain polysaccharide serogroup A/C meningococcal vaccine (MenCPS), by use of a B-cell ELISpot assay, would allow the differentiation of the B-cell characteristics related to immediate antibody production from those specific to immunologic priming.
Patients, materials, and methods
Subjects and vaccines
Volunteers were recruited to a phase 4, open-label, randomized comparison of the memory response of adolescents previously vaccinated with a MenCC (Menjugate; Chiron Vaccines, Marburg, Germany).4 Subjects (age range, 13-15 years) were immunized between 1999 and 2001 as part of the United Kingdom catch-up campaign associated with the introduction of MenCC vaccine into the routine infant immunization schedule. After informed consent by the parent and assent by the child, participants were randomly assigned to receive either MenCPS (Meningivac A + C; Pasteur Merieux, Maidenhead, United Kingdom) or MenCC by intramuscular injection into the deltoid muscle. A 0.5-mL dose of MenCC (containing 10 μg each of CRM197 and serogroup C meningococcal polysaccharide) and a 0.1-mL dose of MenCPS (containing 10 μg of serogroup C meningococcal polysaccharide only) were used. Ethical approval was obtained from the Research Ethics Committees of Oxfordshire (approval number CO2.328) and Mid and South Buckinghamshire (approval number NC1165/703). The trial is registered with clinicaltrials.gov, identifier NCT00262015.
In the phase 4 study, 264 subjects were enrolled in 6 groups to allow investigation of the kinetics of the antibody response between day 0 and day 7 and assessment of overall immunogenicity at day 28 while minimizing the number of blood draws from any one child.12 Groups 1 to 4 received MenCPS, and groups 5 and 6 received MenCC. The blood sampling schedule is shown in Table 1.
Because of the kinetics observed during the initial phase of this study, 40 additional subjects were recruited. Of these subjects, 20 received MenCC and were sampled at days 0, 4, 6, and 28 after immunization (group 7), and 10 each received either MenCC or Men CPS and were sampled on 2 days (either days 8 and 10 or days 9 and 12) after vaccination in addition to day 0 and day 28. Another blood sampling visit was conducted 1 year after immunization with MenCC or MenCPS to assess persistence of the antibody.
The availability of samples for B-cell studies was determined by practical constraints including the availability of a blood sample, the volume of blood collected, the time from venipuncture to arrival in the laboratory (only samples taken less than 12 hours from the time of collection were studied), and time of day at arrival in the laboratory.
Serogroup C meningococcal ELISA
Serum was separated from 5 mL blood and was frozen at -80°C before testing for serogroup C meningococcal polysaccharide-specific antibody concentration by use of standard ELISA methods at the laboratories of Chiron Vaccines.
Preparation of PBMCs
A maximum volume of 5 mL heparinized blood was available for the separation of peripheral blood mononuclear cells (PBMCs). The blood was diluted 1:2 with RPMI 1640 medium (Sigma-Aldrich; Poole, United Kingdom) to which penicillin/streptomycin solution (Sigma-Aldrich) and 200 mM l-glutamine (Sigma-Aldrich) had been added at a dilution of 1:100 (complete medium). PBMCs were then separated by density-gradient centrifugation over Lymphoprep (Axis-Shield, Oslo, Norway). PBMCs were washed once in complete medium before further preparation for ELISpot or cell culture.
Preparation of ELISpot plates
MultiScreen IP 96-well filter plates (Millipore, Bedford, MA) were coated with either 5 μg/mL MenC polysaccharide (National Institute for Biological Standards and Control [NIBSC], South Mimms, Hertfordshire, United Kingdom) conjugated to methylated human albumin (NIBSC), 10 μg/mL diphtheria toxoid (Statens Serum Institut, Copenhagen, Denmark), or 10 μg/mL goat anti-human immunoglobulin (Caltag Laboratories, Burlingame, MA) in sterile PBS. PBS alone was added to the antigen-blank wells. ELISpot plates were stored at 4°C until use.
Detection of plasma cells
PBMCs prepared from peripheral blood were washed 3 times in complete medium with 10% fetal calf serum (Sigma-Aldrich) and resuspended to a final concentration of 2 × 106 PBMC/mL. One hundred microliters per well of the suspension was added to ELISpot plates precoated with MenC polysaccharide in duplicate, as described, and incubated overnight at 37°C in 5% CO2. Antibody-secreting cells (ASCs) were detected with a 1:5000 dilution of goat anti-human immunoglobulin G (IgG) γ-chain-specific alkaline phosphatase conjugate (Calbiochem-Novabiochem, Nottingham, United Kingdom) in complete medium with 10% fetal calf serum, developed using 5-bromo-4-chloro-3-indolyl phosphate in nitroblue tetrazolium dissolved in aqueous dimethylformamide (Bio-Rad Laboratories, Hercules, CA).
Detection of memory B cells
PBMCs prepared from peripheral blood were resuspended in complete medium with 10% human AB serum (Sigma-Aldrich) at a final concentration of 2 × 106 PBMC/mL and were added to 96-well round-bottom culture plates (Costar, Corning, NY) together with a further 100 μL complete medium with 10% human AB serum and 1:5000 Staphylococcus aureus Cowan (Calbiochem, San Diego, CA) and 20 ng/mL IL-2 (Roche Diagnostics, Mannheim, Germany). Cells were incubated at 37°C in 5% CO2 for 5.5 days before resuspension and were washed 4 times in complete medium with 10% fetal calf serum. Cultured cells were plated onto precoated ELISpot plates at 2 × 105 cells/well and then were incubated and developed as described for the ex vivo ELISpots. Duplicate wells were used for each antigen. For the 1-year follow-up samples, it was expected that cell frequencies would be low; hence, at least 5 wells were used for ELISpot wells coated with MenC polysaccharide.
ELISpot counting
Spots were counted using an AID ELISpot Reader System (AID Autoimmun Diagnostika, Strassberg, Germany). Identical settings were used for all plates and antigens, and the operator was blinded to the sample being counted.
Statistical analysis
For the cultured B-cell ELISpots, samples were excluded from the analysis in which fewer than 750 IgG-secreting cells were detected per 2 × 105 cultured lymphocytes, to exclude assays with failed memory B-cell culture. For the purposes of analysis, ELISpot assays in which fewer than 4 spots were detected were treated as though no ASCs were detected. Hence, for postimmunization days 0 to 28, the sensitivity of the assay was 2 ASCs per 2 × 105 lymphocytes from culture. For the 1-year follow-up samples, for which additional wells were used on the ELISpot plate, the minimum sensitivity was 0.9 ASCs per 2 × 105 lymphocytes from culture. All data were entered into spreadsheets (Excel; Microsoft, Redmond, WA). Statistical analysis was undertaken using Stata (version 9.1; StataCorp, College Station, TX). Antibody levels were log transformed and expressed as geometric mean concentrations with 95% confidence intervals (95% CIs). For B-cell numbers, medians were calculated. Between-group comparisons (MenCC vs MenCPS) were made using the Mann-Whitney U test. Within-group comparisons of B-cell numbers at varying time points were made using the Wilcoxon signed-rank test for paired data.
Results
Recruitment
Between September 2003 and June 2004, 303 subjects completed the phase 4 booster study up to 28 days after immunization (including the additional subjects for whom the decision to recruit was made after the onset of the study). Samples from 270 of these subjects were obtained for B-cell studies. In addition, samples were obtained from 38 subjects from 1 year after vaccination to assess persistence of the B-cell responses.
MenC IgG antibody response to vaccination
Before vaccination, the geometric mean MenC antibody concentration was 3.2 μg/mL (95% CI, 2.5-4.1 μg/mL), with no difference at baseline between the group receiving MenCC and that receiving MenCPS. At day 28 after immunization, the geometric mean antibody concentration was significantly greater than baseline for both the MenCC and the MenCPS groups, though the concentration was greater after MenCC immunization (35.7 μg/mL; 95% CI, 29.5-43.2 μg/mL) than after MenCPS immunization (19.5 μg/mL; 95% CI, 17.1-22.34 μg/mL).
Plasma cells
Before vaccination, no MenC-specific cells were detected in peripheral blood in 18 of 19 subjects (Figure 1). In the subject in whom ASCs were detected before immunization, frequency was at the limit of assay detection (2 per 2 × 105 PBMCs). After immunization, the timing of the appearance of MenC-specific ASCs was similar for the MenCC and the MenCPS groups. No MenC-specific ASC response was seen before day 5. Peak ASC responses were seen from day 6 to day 8 and were followed by a rapid decline in the number of samples with any detectable ASC response over days 9 to 12. By day 7, the subjects given MenCC had a greater frequency of MenC-specific ASCs than those given MenCPS (104 vs 34.75 ASCs/2 × 105 PBMCs; P = .001). At day 12, MenC-specific ASCs were almost undetectable, and no cells could be identified at day 28.
Correlation with antibody responses
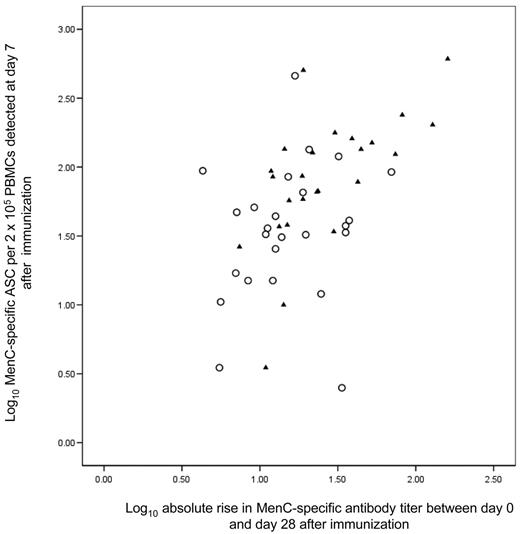
Overall, after adjusting for the vaccine group, there was a significant correlation between the number of MenC ASCs on day 7 and the absolute increase in MenC antibody level from prevaccination to day 28 (r = 0.43; P = .001).However, when considered for each vaccine group separately, the correlation was significant only for those given MenCC (r = 0.58; (P < .001) and not for the MenCPS group (r = 0.22; P = .27) (Figure 2).
Memory B cells
Before vaccination, only 3 of 37 subjects had detectable MenC-specific memory B cells. In view of the small numbers, a correlation with resting antibody concentration was not possible. The first response in relation to baseline was seen by day 6 for the MenCC group and day 7 for the MenCPS group (Figure 3). At this time, similar proportions of subjects in the MenCC and the MenCPS groups had detectable memory B cells. However, the magnitude of response was greater in the MenCC group (12.5 vs 2.75 ASCs per 2 × 105 cultured lymphocytes). By 1 month of age, in a subset of subjects for whom paired data were available, the number of subjects with detectable MenC-specific B cells in the MenCPS group was no different from baseline, with a median value of zero MenC-specific memory B cells per 2 × 105 cultured lymphocytes (P = .66). In contrast, there was a persistent response for the MenCC group, with a median of 23 MenC-specific memory B cells (P = .002).
Plasma cell response to immunization. (A) MenC polysaccharide vaccine. (B) MenC polysaccharide/CRM197 conjugate vaccine. Subjects were immunized on day 0, and PBMCs were separated on various days after immunization. Numbers of subjects whose PBMCS were obtained for ELISpot for each day after immunization are given in the tables below each panel. Different children were studied at each time point. B-cell numbers are expressed as MenC-specific ASCs per 2 × 105 PBMCs. Horizontal bars represent the median number of MenC-specific ASCs at each time point. Blank cells indicate days on which no blood samples were taken.
Plasma cell response to immunization. (A) MenC polysaccharide vaccine. (B) MenC polysaccharide/CRM197 conjugate vaccine. Subjects were immunized on day 0, and PBMCs were separated on various days after immunization. Numbers of subjects whose PBMCS were obtained for ELISpot for each day after immunization are given in the tables below each panel. Different children were studied at each time point. B-cell numbers are expressed as MenC-specific ASCs per 2 × 105 PBMCs. Horizontal bars represent the median number of MenC-specific ASCs at each time point. Blank cells indicate days on which no blood samples were taken.
Correlation of log 10 MenC-specific plasma cell response with log 10 absolute increase in MenC antibody response. Data points marked by ▴ represent individuals who received MenCC; those with ○, individuals who received MenCPS. Pearson correlation coefficient was 0.58 for the MenCC group (P < .001) and 0.22 for the MenCPS group (P = .27).
Correlation of log 10 MenC-specific plasma cell response with log 10 absolute increase in MenC antibody response. Data points marked by ▴ represent individuals who received MenCC; those with ○, individuals who received MenCPS. Pearson correlation coefficient was 0.58 for the MenCC group (P < .001) and 0.22 for the MenCPS group (P = .27).
By 1 year of age, the MenC-specific memory B-cell numbers in the MenCC group remained significantly elevated in relation to the MenCPS group (P < .011; median, 1.25 vs 0.0 MenC-specific memory B cells per 2 × 105 cultured lymphocytes). The median for the MenCPS group was the same as at baseline and at 1 month.
Discussion
This is the first study to compare the magnitude and the kinetics of plasma and memory B-cell responses to a polysaccharide and a glycoconjugate vaccine. The induction of long-term protection against invasive diseases caused by encapsulated bacteria, such as the meningococcus, can be achieved through the induction of persistent serum antibodies to capsular polysaccharide. In adolescents, antibody persists for at least 3 years after MenC glycoconjugate vaccination.4,12 Concentrations of these antibodies are sustained by long-lived plasma cells and persistence of memory B cells.13 When given in the first 2 years of life, antibody levels decline more rapidly.5,12,14 Immunologic priming for an enhanced secondary response occurs when the vaccine is given in infancy or later in life.4,5,14 Understanding the mechanism by which vaccines generate and sustain these B-cell populations is likely to be important in explaining and predicting the long-term efficacy of vaccines that mediate their protection through antibody.
In this study of children previously primed with MenCC, we have shown that both a glycoconjugate vaccine (MenCC) and a plain polysaccharide vaccine (MenCPS) generate MenC-specific plasma cells. Although for both vaccines the timing of appearance of plasma cells in the peripheral blood is identical (5-10 days after immunization), MenCC generated a greater response than MenCPS by day 7 (104 vs 35 MenC ASCs/2 × 105 PBMCs). However, there were more striking differences in the memory B-cell responses. MenCPS was associated with a small and transient increase in MenC-specific memory B cells by day 7 (median, 2.75 MenC memory B cells/2 × 105 cultured PBMCs) that no longer differed from baseline by 1 month after immunization. In contrast, MenCC vaccination induced a greater number of memory B cells by day 7 after immunization (12.5 MenC memory B cells/2 × 105 cultured PBMCs). At 1 year after immunization with MenCC, the memory B-cell response was significantly greater than in the MenCPS group. We suggest that this persistence of circulating memory B cells reflects differences in the ability of these vaccines to induce immunologic priming.
Plasma cell responses
The ex vivo B-cell ELISpot assay detects cells able to spontaneously secrete antibody immediately after the isolation of PBMCs. Previous studies indicate that the phenotype for these cells is consistent with that of plasma cells—CD27+CD20-CD38hi.15 In addition, CD38hiCD19+ B cells secreting antibody directly after isolation from peripheral blood have been shown by electron micrography to have the phenotype of plasma cells.16
Memory B-cell response to immunization. (A) MenC polysaccharide vaccine. (B) MenC polysaccharide/CRM197 conjugate vaccine. Subjects were immunized on day 0, and PBMCs were separated on various days after immunization and cultured with SAC/IL2 for 5.5 days before MenC-specific ELISpot assays. Numbers of subjects whose PBMCS were obtained for ELISpot for each day after immunization are given in the table below the chart. Different subjects were studied at each time point. B-cell numbers are expressed as MenC-specific ASCs per 2 × 105 cultured lymphocytes. Horizontal bars represent the median number of MenC-specific ASCs at each time point. Blank cells are as in Figure 1.
Memory B-cell response to immunization. (A) MenC polysaccharide vaccine. (B) MenC polysaccharide/CRM197 conjugate vaccine. Subjects were immunized on day 0, and PBMCs were separated on various days after immunization and cultured with SAC/IL2 for 5.5 days before MenC-specific ELISpot assays. Numbers of subjects whose PBMCS were obtained for ELISpot for each day after immunization are given in the table below the chart. Different subjects were studied at each time point. B-cell numbers are expressed as MenC-specific ASCs per 2 × 105 cultured lymphocytes. Horizontal bars represent the median number of MenC-specific ASCs at each time point. Blank cells are as in Figure 1.
Studies of immunized human subjects have detected antigen-specific plasma cell responses at 6 to 9 days after immunization with several different immunogens.11,15-17 The number of antigen-specific plasma cells that can be detected in peripheral blood declines rapidly after this to the very low frequencies found before vaccination. This is a consistent feature across many studies using a variety of antigens, including polysaccharides. Some of these studies have demonstrated a weak correlation between the increase in antibody level after vaccination and the peak plasma cell response.11 The transient appearance of plasma B cells in peripheral blood is thought to represent the passage of newly formed plasma cells from their site of production in the lymphoid tissue to more long-term residence in the bone marrow. The increase in antibody levels after immunization is attributed to immunoglobulin production by these newly formed plasma cells—hence, the correlation between these 2 variables.
There was a significant correlation between the number of ASCs on day 7 and the absolute increase in antibody concentration from day 0 to day 28 for the MenCC group (r = 0.58; P < .001) but no correlation for the MenC PS group (r = 0.22; P = .27). The lack of correlation for the MenCPS vaccine might have been related to the lower magnitude of plasma cell response. More children in that group had cell frequencies that were closer to the limit of detection and were therefore less accurately estimated.
These results suggest that the initial increase in antibody titer is likely to be directly related to the plasma cells detected in the ex vivo ELISpot assay. It is unclear, with the current methodology, whether any of the plasma cells generated by vaccination may be long-lived and may contribute significantly to sustaining long-term antibody production. Such a population might represent only a small fraction of the total numbers of cells generated. In addition, it is possible that it is the availability of bone marrow niches rather than cell phenotype that is important in dictating the proportion of cells that become long-lived.18 Hence, measuring plasma cell output alone after vaccination would not enable an estimation of long-term antibody persistence.
In our study, immunization with both MenCPS and MenCC resulted in the presence of detectable plasma cells between days 5 and 9. Previous studies have demonstrated that the covalent bonding of CRM197 to MenC polysaccharide in MenCC results in a vaccine antigen able to elicit T-cell help for B cells as opposed to MenCPS, which can only stimulate B cells independently of T cells. Thus, with respect to a secondary antibody response in this study, it is striking that the presence (MenCC) or absence (MenCPS) of cognate T-cell help in conjunction with MenC polysaccharide did not alter the appearance of plasma cells in the blood. This is consistent with the derivation of plasma cells from previously generated memory B cells. These cells apparently did not require T-cell help to begin proliferation and differentiation into plasma cells8 because the presence of a single signal, the MenC polysaccharide oligomer, was sufficient to activate memory B cells for either vaccine. Although the appearance of plasma cells after vaccination with MenCC was almost identical to that of MenCPS vaccine, the magnitude of the MenCC plasma cell response was almost 3 times greater than that of the plain polysaccharide (125 vs 35 ASC/2 × 105 PBMCs on day 7). Both vaccines contained the same amount of MenC polysaccharide (10 μg). Although both vaccines may trigger a memory response equally rapidly (because of the sufficiency of a single signal to the memory B cell), it appears that specific T-cell help acts as an additional stimulus to the degree of expansion of the memory B-cell population into plasma cells.
Memory B-cell response
The use of polyclonal B-cell activators in culture stimulates the CD27+ B-cell memory population to proliferate and secrete antibody, thereby allowing their detection by ELISpot methods.19 Although the CD27+ phenotype suggests that naive cells are not detected by these assays, it does not exclude the possibility that CD27+ plasma cells, present in the PBMCs when first isolated, are also detected. However, other investigators have shown that in cell culture, plasma cells do not survive for more than a few days without appropriate supporting stromal cells (eg, fibroblasts).20 Thus cell culture in the context of this study with polyclonal stimulation for 5 days followed by B-cell IgG ELISpot is likely to be specific for the detection of CD27+ memory B cells alone.
Previous studies demonstrate antigen-specific memory B cells from the end of the first week after vaccination with protein antigens.7,21 After the initial response, the numbers of antigen-specific cells decline to a plateau level over several months; subsequently, antigen-specific memory B cells can be detected in most people for many years.6,7,19,22,23 In a group of previously vaccinated adults, memory B cells were detected for diphtheria at 0.01% to 1% of total IgG-secreting memory B cells and for tetanus at 0.1% to 1% of total IgG-secreting memory B cells several years after vaccination.7 In another study, subjects given smallpox vaccination had memory B-cell frequencies of approximately 0.1% of total IgG-secreting memory cells for several decades after vaccination.6,7 In an earlier study, tetanus-specific memory B cells were present at a mean frequency of 15.7 per 106 cultured PBMCs at a mean interval of 10 years since vaccination. There was no comparison with total IgG-secreting cells to allow the percentage to be calculated.21 The mechanisms by which memory B cells are involved in antibody persistence are still unclear. In some cases a correlation has been noted between the frequency of circulating memory cells and antibody levels,6,15,22 with the suggestion that circulating levels of antibody are in proportion to total memory B-cell numbers, which in turn are reflected by circulating B-cell numbers. Other investigators have found no such relation.17 It is unknown to what extent memory B-cell responses immediately after vaccination reflect the generation of new memory B cells rather than the stimulation and recirculation of preexisting memory cells.
In the current study, the mean lower limit of detection of antigen-specific memory B cells in the ELISpot assay was 0.05% of total IgG-secreting memory cells after culture. In only 3 of 37 subjects were significant numbers of MenC-specific memory cells detected in the peripheral blood at baseline, despite all volunteers having been primed with MenCC 2 to 3 years earlier. The reported frequency of memory B cells of 0.01% to 0.1% is below the limit of sensitivity for our assay using small blood volumes in subjects. Because the proportion of subjects with detectable MenC memory cells was low, it was not possible to correlate the cell numbers with the resting antibody titers.
The current study demonstrates marked differences in memory B-cell responses after immunization with MenCC compared with MenCPS. These differences were consistent with the T cell-dependent and -independent characteristics of MenCC and MenCPS, respectively. Both vaccines induced the appearance of MenC-specific memory B cells by day 7 after immunization. The difference in magnitude of response (MenCC greater than MenCPS) was similar to that seen for the plasma cell response. However, by 1 month after immunization, the difference between the 2 groups was marked, with a median of zero memory B cells detected for the MenCPS group but a striking persistence of B cells in the MenCC group. Memory B cells are still significantly elevated at 1 year after MenCC immunization in comparison to MenCPS immunization, though the magnitude of response is much diminished.
It is unclear whether the memory B cells detected in peripheral blood are derived from newly stimulated naive B cells proliferating in germinal centers or from the proliferation of memory cells laid down after the original priming MenCC vaccination. However, given the absence of immunologic priming with MenCPS indicated by antibody studies, we hypothesize that the marked difference in memory B-cell responses between the 2 vaccines was related to the production of new antigen-specific B-cell memory. Although germinal centers persist for only approximately 3 weeks after immunization, memory B-cell blasts continue to proliferate in the follicles of lymphoid tissue for months, suggesting a mechanism whereby a continued high level of circulating memory B cells might be maintained.24
Consistent with other T cell-independent polysaccharide antigens, MenCPS does not prime for a secondary immune response. However, unlike many other T cell-independent antigens, MenC polysaccharide vaccine is well known to induce hyporesponsiveness in adults and children. After the first dose, subsequent doses of vaccine provide diminished antibody response.25,26 As a result, the detection of any B-cell memory response to MenCPS by day 7 is notable. Although it is likely that the memory B cells detected in response to the MenCC vaccine are newly generated in germinal centers, it is possible that a proportion are extant memory cells from previous antigen exposure that are recirculating in response to the new antigenic stimulus. An explanation for the immunologic hyporesponsiveness that has been attributed to the use of plain MenC polysaccharide vaccines is that mobilization of extant memory B cells without regeneration of a memory cell population diminishes the total antigen-specific memory cell pool. On reencounter, a smaller population of cells responds rapidly to the polysaccharide, and a lower level of antibody is generated.
In conclusion, this is the first study to directly show that, in contrast to plain polysaccharide meningococcal vaccines, a MenC glycoconjugate vaccine induced the generation of antigen-specific memory B-cell populations that persisted for up to 1 year after a booster dose of MenC vaccine. Detection of peripheral blood memory B-cell responses after vaccination may be a useful signature of successful induction of immunologic memory during novel vaccine evaluation. Additional studies are now necessary using these techniques to establish the optimal dosing schedules to provide sustained protection against such polysaccharide-encapsulated bacteria with the aim of further reducing childhood mortality.
Prepublished online as Blood First Edition Paper, May 4, 2006; DOI 10.1182/blood-2006-01-009282.
Supported by Chiron Vaccines.
Several authors (A.J.P., E.R.M.) have conducted clinical trials on behalf of Oxford University that were sponsored by a company (Chiron Vaccines) or a competitor (Wyeth, GlaxoSmithKline, Sanofi Pasteur, Sanofi Pasteur MSD) of a company whose potential product was studied in the present work. One of these authors (A.J.P.) does not hold any paid consultancies with vaccine manufacturers, but industry-sourced honoraria for consultancy, writing, or lecturing are paid to educational funds in the department of pediatrics or to an independent charity. One of the authors (E.R.M.) is a member of the Scientific Advisory Board of a company (Chiron Vaccines) whose potential product was studied in the present work. One of the authors (A.B.) was an employee of a company (Chiron Vaccines) whose potential product was studied in the present work. One of the authors (M.D.S.) has received assistance to attend scientific meetings of a company (Chiron Vaccines) whose potential product was studied in the present work.
D.F.K. planned the study, conducted laboratory work, analyzed the data, and wrote drafts of the paper. M.D.S. conducted clinical trial work and reviewed drafts of the paper. E.A.C. developed the laboratory methodology, conducted laboratory work, interpreted results, and reviewed drafts of the manuscript. S.G. and C.S. administered the clinical trial on a daily basis and collected all clinical samples. L.D. conducted the overall administration of the clinical trial. L-m.Y. conducted statistical analysis of the data. A.B. helped design the study. E.R.M. helped design the study and interpret the data. A.J.P. was the principal investigator, discussed and planned clinical and laboratory aspects of the study, analyzed data, and reviewed drafts of the paper. All authors reviewed the final draft of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.