Comment on Modlich et al, page 2545
In this issue of Blood, Modlich and colleagues report using a novel culture assay to demonstrate a self-inactivating (SIN) retrovirus vector design that can significantly reduce, but not eliminate, vector-mediated transformation of primary mouse bone marrow cells.
A small but growing number of clinical trials are proving the potential of gene therapy as a viable approach for the treatment of inherited and acquired hematologic diseases. These trials owe their success in large part to the use of gene transfer vectors based on recombinant retroviruses, especially gammaretroviruses, that can efficiently integrate therapeutic genes into the chromosomes of target cell populations such as T cells or hematopoietic stem/progenitor cells. However, as recently reviewed,1,2 with this success has come the revelation of a dose-limiting toxicity related to the ability of these vectors to activate cellular oncogenes located near sites of vector integration. This permutation of vector-related genotoxicity has typically been attributed to the potent enhancers or, to a lesser extent, promoters located in the virus long terminal repeats (LTRs) (see figure). The offending sequences can be removed by deleting the U3 region of the virus LTR, which results in a self-inactivating, or SIN, vector design. However, this approach requires the placement of promoters/enhancers within the body of the vector in order to express the therapeutic gene.
In this issue of Blood, Modlich and colleagues report a series of studies investigating whether a SIN vector design coupled with a single internal U3 promoter/enhancer reduces the risk of genotoxicity compared with a conventional non-SIN vector. The authors also report the development of a novel method for assessing the rate of vector-mediated genotoxicity. Specifically, this approach involves expanding pools of transduced lin- mouse bone marrow cells in bulk culture for 2 weeks, and then measuring the rate with which individual clones continue to grow following limiting dilution. This approach has several advantages, including the use of primary cells, the requirement for self-renewal and growth competition, and the rapidity of the assay. However, the use of an extensive bulk-culture phase limits the ability of this approach to quantify in absolute terms the frequency of transformation, and the resulting clones were not assessed in vivo for tumor formation. In addition, the fact that most transformation events were associated with the activation of one specific oncogene (Evi1) suggests a limit to the potential transformation targets that can be detected. Nevertheless, these studies show a SIN-based vector design can decrease by 12-fold the rate of vector-mediated transformation, strongly supporting the use of the SIN vector design in clinical gene transfer trials involving the use of retrovirus vectors. It is also worth noting that the transformation rate associated with the SIN vector remained surprisingly high—1 in 4347 transduction events—indicating the need for studies into additional methods of reducing the interaction between vector elements and the surrounding genome, such as the use of alternative recombinant viruses3 or chromatin insulators.4 ▪
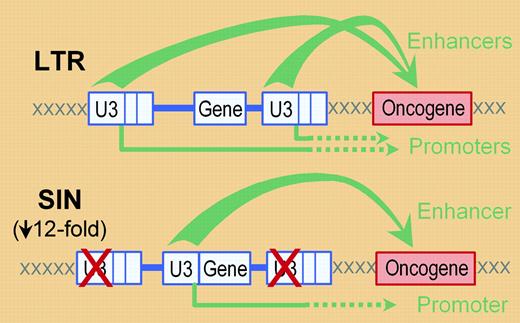
A typical retrovirus vector (in blue) can activate a cellular oncogene (in red) through either the enhancers (top arrows) or promoters (bottom arrows) present in the U3 regions of the virus long terminal repeats (LTRs). Generation of a self-inactivating (SIN) vector by deletion of the U3 regions in the vector LTRs and inclusion of an internal enhancer/promoter (the U3 region in this case) should reduce, but not eliminate, these potential interactions. Modlich and colleagues show that these changes reduce the rate of vector-mediated transformation by approximately 12-fold (based on the replating efficiencies of transduced bone marrow). Note that enhancers can also activate oncogenes in the 5′ direction (not shown).
A typical retrovirus vector (in blue) can activate a cellular oncogene (in red) through either the enhancers (top arrows) or promoters (bottom arrows) present in the U3 regions of the virus long terminal repeats (LTRs). Generation of a self-inactivating (SIN) vector by deletion of the U3 regions in the vector LTRs and inclusion of an internal enhancer/promoter (the U3 region in this case) should reduce, but not eliminate, these potential interactions. Modlich and colleagues show that these changes reduce the rate of vector-mediated transformation by approximately 12-fold (based on the replating efficiencies of transduced bone marrow). Note that enhancers can also activate oncogenes in the 5′ direction (not shown).