Abstract
Somatic activation of a conditional targeted KrasG12D allele induces a fatal myeloproliferative disease in mice that closely models juvenile and chronic myelomonocytic leukemia. These mice consistently develop severe and progressive anemia despite adequate numbers of clonogenic erythroid progenitors in the bone marrow and expanded splenic hematopoiesis. Ineffective erythropoiesis is characterized by impaired differentiation. These results demonstrate that endogenous levels of oncogenic Ras have cell lineage-specific effects and support efforts to modulate Ras signaling for therapy of anemia in patients with myelodysplastic syndromes and myeloproliferative disorders.
Introduction
Anemia with histopathologic and laboratory evidence of ineffective erythropoiesis is a hallmark of myelodysplastic syndromes (MDSs) and of juvenile and chronic myelomonocytic leukemia (JMML and CMML), which are classified as MDS/myeloproliferative disorder (MDS/MPD) overlap diseases.1,2 In addition to monocytic myeloproliferation, JMML and CMML are characterized by anemia with splenomegaly, extramedullary hematopoiesis, and variable degrees of erythroid dysplasia.3-6
Deregulated signaling pathways play a fundamental role in the pathogenesis of myeloid malignancies.7 Mutations in NRAS or KRAS2 are among the most common genetic lesions found in acute myeloid leukemia (AML), MDS, and MPD. Ras proteins are signal switch molecules that regulate growth and differentiation by cycling between an active guanosine triphosphate (GTP)–bound state (Ras-GTP) and an inactive guanosine diphosphate (GDP)–bound state. Oncogenic Ras proteins accumulate in the GTP-bound conformation due to defective intrinsic GTP hydrolysis and resistance to GTPase activating proteins (GAPs).8-10 In addition to oncogenic RAS mutations, Ras signaling is deregulated in myeloid malignancies by alternative genetic mechanisms that include the BCR-ABL fusion, PTPN11 mutations, and NF1 inactivation.11-13
Expressing oncogenic RAS alleles with retroviral vectors inhibits differentiation of erythroid progenitors in vitro, suggesting that hyperactive Ras has a direct pathogenic role in the anemia that occurs in MDS and in MDS/MPD overlap disorders.14,15 However, retroviral transduction requires ex vivo manipulation of primary cells and results in supraphysiologic Ras protein levels that could contribute to these phenotypes.16 Somatic activation of oncogenic KrasG12D results in a fatal monocytic MPD in Mx1-Cre, KrasG12D mice that is associated with anemia and splenomegaly with extramedullary hematopoiesis.17,18 Here we show that expressing KrasG12D from its endogenous promoter induces ineffective erythropoiesis in vivo, with an apparent block in differentiation at the proerythroblast stage.
Study design
Mice
Mice were maintained in the sterile UCSF animal care facility and were fed pelleted chow and acidified water ad libitum. The experimental procedures were approved by the UCSF Committee on Animal Research. Breeding, genotyping, polyinosinic-polycytidylic acid (pIpC) injection and hematologic studies were performed as described.17
EPO levels and flow cytometry
Serum erythropoietin (EPO) was measured by enzyme-linked immunosorbent assay (Quantikine MEP00, R&D Systems, Minneapolis, MN). Staining for CD71, TER119, 5′-bromodeoxyuridine (BrdU), and with annexin V and 7-amino-actinomycin D (7-AAD) were performed using commercial fluorochrome-conjugated reagents following manufacturer's instructions (BD Pharmingen, San Diego, CA). Mice were given intraperitoneal injections of BrdU (150 mg/kg) 1 hour prior to harvesting tissue for analysis.
In vitro cultures
Erythroid differentiation assays were performed according to published methodologies14 with the addition of a 4-day preincubation in IL-11 (100 ng/mL), SCF (100 ng/mL), Flt-3 (50 ng/mL), IL-6 (20 ng/mL), and IFN-β (400 U/mL; all from R&D Systems) to induce Mx1-Cre expression. Progenitor colonies were enumerated in methylcellulose medium (M3234; StemCell Technologies, Vancouver, BC, Canada) supplemented with recombinant human EPO (R&D Systems) and counted after incubation for 2 to 3 days (erythroid colony-forming units [CFU-Es]) or 7 days (erythroid burst-forming units [BFU-Es]). Photomicrographs were taken using a TMS-F inverted microscope fitted with a 10×/0.25 objective, Ph3 annulus, and Coolpix 5000 digital camera (Nikon, Melville, NY).
Serum EPO and erythroid progenitors in Mx1-Cre, KrasG12D mice. (A) Serum EPO and hemoglobin (Hb) concentrations in Mx1-Cre, KrasG12D mice (open symbols) and wild-type (wt) littermates (closed symbols); data are pooled from F1 (n = 18; circles) and C57BL/6 (n = 9; squares) mice. (B) Total CFU-Es recovered from bone marrow (2 femurs and 2 tibias) and spleens of Mx1-Cre, KrasG12D mice (□) and wild-type littermates (▪) shown as mean ± SEM. (C) Total BFU-Es are enumerated as in panel B. (D) EPO dose-response of BFU-Es derived from C57BL/6 bone marrow, showing mean ± SEM from 3 independent experiments. (E,F) Photomicrographs of typical BFU-Es from bone marrow cultured with 50 ng/mL EPO for 7 days (bar represents 500 μm).
Serum EPO and erythroid progenitors in Mx1-Cre, KrasG12D mice. (A) Serum EPO and hemoglobin (Hb) concentrations in Mx1-Cre, KrasG12D mice (open symbols) and wild-type (wt) littermates (closed symbols); data are pooled from F1 (n = 18; circles) and C57BL/6 (n = 9; squares) mice. (B) Total CFU-Es recovered from bone marrow (2 femurs and 2 tibias) and spleens of Mx1-Cre, KrasG12D mice (□) and wild-type littermates (▪) shown as mean ± SEM. (C) Total BFU-Es are enumerated as in panel B. (D) EPO dose-response of BFU-Es derived from C57BL/6 bone marrow, showing mean ± SEM from 3 independent experiments. (E,F) Photomicrographs of typical BFU-Es from bone marrow cultured with 50 ng/mL EPO for 7 days (bar represents 500 μm).
Results and discussion
Mx1-Cre, KrasG12D mice develop fatal monocytic MPD on both F1 C57BL/6 × 129Sv/Jae and inbred C57BL/6 backgrounds (Braun et al17 and data not shown). Although myeloid proliferation is modestly attenuated in C57BL/6 mice, there is no difference in time of onset or severity of anemia in these strains. Blood smears show polychromasia and reticulocytosis, with hypochromia and anisopoikilocytosis in moribund animals. Serum EPO levels are elevated in proportion to anemia, consistent with the histopathologic features suggestive of ineffective erythropoiesis (Figure 1A).
We enumerated erythroid progenitor colonies from Mx1-Cre, KrasG12D bone marrow and spleen cells in methylcellulose medium. Surprisingly, although erythroid lineage cells were extremely rare in cytologic preparations of Mx1-Cre, KrasG12D marrow (data not shown), we detected normal numbers of BFU-E and CFU-E progenitors (Figure 1B-C). BFU-Es from Mx1-Cre, KrasG12D marrow formed abnormally large colonies that were characterized by both EPO-independent growth and hypersensitivity to EPO (Figure 1D-F). Furthermore, the enlarged spleens from Mx1-Cre, KrasG12D mice contained abundant BFU-Es and CFU-Es (Figure 1B-C), with similarly enlarged BFU-E colonies. Thus, the erythroid progenitor compartment is massively expanded in Mx1-Cre, KrasG12D mice despite severe anemia. Together with the elevated serum EPO levels and severe reduction in differentiated erythroid precursors, these data support the idea that impaired terminal erythroid development contributes to anemia. These data also underscore the similarity of MPD in Mx1-Cre, KrasG12D mice to JMML, which is characterized by BFU-Es that exhibit a degree of spontaneous growth as well as growth factor hypersensitivity.6,19
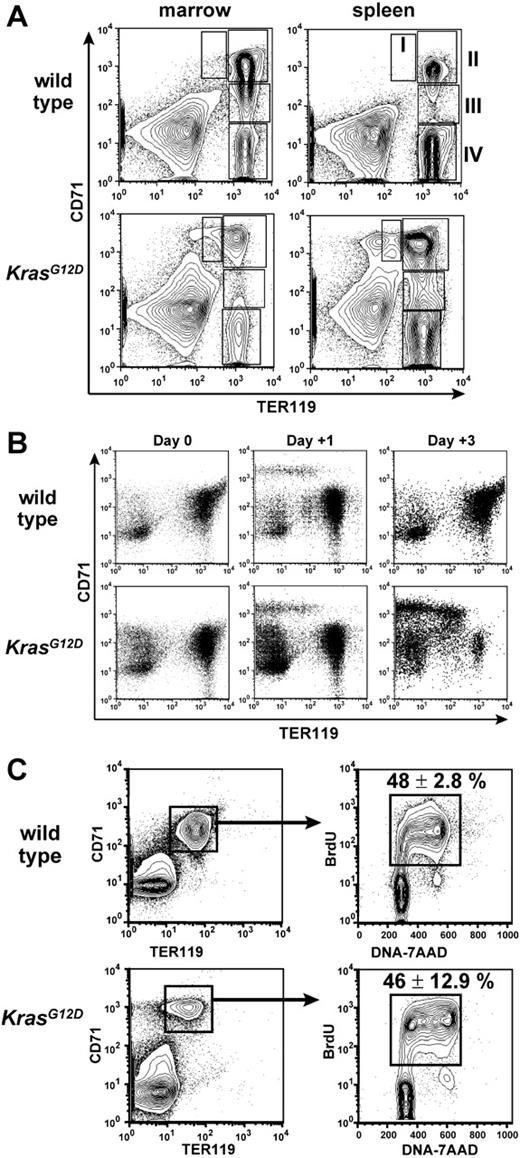
Differentiation and proliferation of erythroid progenitors. (A) Flow cytometry analysis of CD71 and TER119 expression of freshly harvested hematopoietic cells, with regions of maturing erythroid cells indicated as described.20 Table S1 presents the quantification. (B) CD71 and TER119 expression of E14.5 fetal liver cells after in vitro induction of KrasG12D for 4 days (day 0), then after 1 day of EPO stimulation (day +1), or after 2 days of EPO stimulation and 1 day of EPO withdrawal (day +3). (C) BrdU incorporation in vivo by CD71+TER119+ bone marrow cells. Note that the CD71/TER119 staining profile reflects lysis of erythrocytes and fixation required for BrdU staining. Mean percentage BrdU+ cells ± SEM is shown.
Differentiation and proliferation of erythroid progenitors. (A) Flow cytometry analysis of CD71 and TER119 expression of freshly harvested hematopoietic cells, with regions of maturing erythroid cells indicated as described.20 Table S1 presents the quantification. (B) CD71 and TER119 expression of E14.5 fetal liver cells after in vitro induction of KrasG12D for 4 days (day 0), then after 1 day of EPO stimulation (day +1), or after 2 days of EPO stimulation and 1 day of EPO withdrawal (day +3). (C) BrdU incorporation in vivo by CD71+TER119+ bone marrow cells. Note that the CD71/TER119 staining profile reflects lysis of erythrocytes and fixation required for BrdU staining. Mean percentage BrdU+ cells ± SEM is shown.
We examined bone marrow cells and splenocytes from anemic Mx1-Cre, KrasG12D mice by flow cytometry to investigate later stages in erythroid differentiation (Figure 2A). These studies revealed normal numbers of early erythroid cells in bone marrow (CD71hiTER119–/low, corresponding to proerythroblasts and some CFU-Es20 ), but a paucity of all the more mature TER119hi populations (regions II-IV, Figure 2A, and Table S1, which is available on the Blood website; see the Supplemental Table link at the top of the online article). This suggests an inefficient transition from TER119– to TER119hi stages of erythropoiesis. Supporting this idea, spleens of Mx1-Cre, KrasG12D mice also contained an excess of immature CD71hiTER119–/low cells. However, these spleens also harbored large numbers of TER119hi erythroblasts, which suggests that the splenic microenvironment partially mitigates the erythropoietic defects caused by hyperactive Ras. The abundance of TER119hi erythroblasts in spleens of Mx1-Cre, KrasG12D mice also indicates that inefficient production of CD71+TER119hi cells from CFU-Es does not fully explain the anemia. This is consistent with studies of human MDS, which imply multifactorial etiologies of anemia.1 The overrepresentation of immature erythroid cells was recapitulated in recipients given transplants of Mx1-Cre, KrasG12D bone marrow (n = 2) or splenocytes (n = 3), verifying that this phenotype is intrinsic to the hematopoietic compartment. Finally, similar cellular distributions were noted when Mx1-Cre, KrasG12D fetal liver cells were induced to undergo erythroid differentiation in vitro (Figure 2B), demonstrating a significant cell intrinsic component to the differentiation block.
We gave Mx1-Cre, KrasG12D mice injections of BrdU to determine the percentage of cells in the S phase of the cell division cycle. The CD71hiTER119+ fraction demonstrated similarly high rates of proliferation in vivo in wild-type and Mx1-Cre, KrasG12D mice (Figure 2C). Some previous studies of transduced erythroid cells showed that expressing oncogenic Ras proteins increases the percentage of apoptotic cells,15 whereas others did not.14 To address this question, we assayed freshly harvested bone marrows and spleens for annexin V staining. Although increased annexin V staining was observed occasionally in CD71hiTER119+ cells from moribund Mx1-Cre, KrasG12D mice, some mice with severe anemia demonstrated wild-type levels of annexin V staining in all erythroid populations (data not shown). These data indicate that excessive apoptosis may occur in erythroblasts of some animals but does not, in general, explain the anemia in Mx1-Cre, KrasG12D mice.
In summary, we find that K-RasG12D causes dyserythropoiesis in vivo in a genetically and phenotypically accurate model of JMML and CMML. This analysis of primary erythroid cells that express oncogenic Kras from its endogenous promoter in the context of an intact organism extend previous in vitro studies. Our data showing impaired differentiation during terminal erythroid maturation are generally consistent with results from retroviral systems; however, the finding that Mx1-Cre, KrasG12D mice sustain erythropoiesis more effectively in spleen than in bone marrow also demonstrates a role for the microenvironment in modulating this effect. Because terminal maturation of erythroid cells is highly dependent on their association with supportive macrophages,21 we speculate that anemia in Mx1-Cre, KrasG12D mice may derive, in part, from effects of hyperactive Ras on macrophage differentiation or function or both.
The Nf1 tumor suppressor gene encodes a GAP that attenuates Ras signaling. Ablating a conditional Nf1 mutant allele induces MPD in Mx1-Cre, Nf1flox/flox mice that is qualitatively similar to the disorder in Mx1-Cre, KrasG12D mice, but more indolent.22 Similarly, expressing a conditional mutant Ptpn11 allele results a mild MPD.23 Interestingly, although splenic erythropoiesis is prominent in both strains, neither develops anemia. Nf1 inactivation and mutant Ptpn11 expression both result in hyperactive Ras signaling; however, Ras-GTP levels are highest in Mx1-Cre, KrasG12D hematopoietic cells. These data, as well as analyses of germline activated KRAS2 alleles,24 support the idea that effects on erythroid differentiation vary with the degree and duration of Ras activation.
We find that K-RasG12D causes anemia, at least in part, through inhibitory effects on erythroid differentiation. Our data support the hypothesis that hyperactive Ras directly contributes to ineffective erythropoiesis and anemia in some patients with MDS and MDS/MPD overlap disorders, in whom transfusion dependence is a major morbidity.25 In vitro data implicate aberrant MEK activity downstream of Ras-GTP in the erythroid differentiation arrest induced by oncogenic Ras.26 Pharmacologic inhibitors of MEK, which are being developed as potential cancer agents, might therefore ameliorate the anemia seen in JMML and CMML, and in some patients with MDS. Mx1-Cre, KrasG12D mice are a robust preclinical platform for testing this new therapeutic strategy.
Prepublished online as Blood First Edition Paper, May 23, 2006; DOI 10.1182/blood-2006-01-013490.
Supported in part by National Institutes of Health grants K08 CA103868, R01 CA72614, and P30 CA082103, and by the Frank A. Campini Foundation.
B.S.B. designed the experiment, performed research, and wrote the manuscript; J.A.A. maintained the mice and performed research; J.A.V.Z. designed the experiment and performed research; D.A.T. and T.E.J. developed the transgenic mice; K.S. designed the experiment and wrote the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
T.E.J. is an investigator of the Howard Hughes Medical Institute.