Beullens et al report the “false-positive detection of recombinant human erythropoietin in urine following strenuous physical exercise.”1(p4711) This report, based on observations conducted on urine from 1 single subject, relies in fact on serious errors of interpretation of poor-quality images. A first sample, collected just after exercise and analyzed by double-blotting following isoelectric focusing of the retentate from ultrafiltrated urine,2,3 gives rise to a banding pattern interpreted as unrelated to Epo based on the argument that this pattern is missing in a second sample collected 1 hour later. A simple routine assay (using other antibodies than the AE7A5 used for immunoblot) of the Epo level in these 2 ultrafiltered samples before IEF would have probably shown that a high concentration of this hormone was present in the first one but not in the second one. It is quite surprising that this basic control was not performed. Did the authors fail to obtain an image of natural urinary Epo for comparison with their “interfering protein”? The SDS electrophoresis results are entirely misinterpreted due to the very different conditions chosen for preparation of the same urine sample before SDS (10-fold concentration) and IEF (200-fold concentration). Under such distorted conditions, the absence of a band corresponding to the molecular weight of Epo (39 kDa) in SDS electrophoresis cannot, in any account, be considered as a proof that there was no EPO in the sample submitted to IEF. The only band detected (42 kDa) by SDS has been incorrectly related to the bands detected by IEF. Why isn't a 2-dimensional electrophoresis shown to demonstrate such an assertion? In fact, the bands shown in the IEF figure cannot be detected in the SDS experiment due to the insufficient concentrating step. From our experience, the 42-kDa band corresponds to a protein very often present in urine in high concentrations after strenuous exercise and detected by the AE7A5 antibody used for immunoblotting. This protein is known not to interfere with the Epo pattern of an antidoping control due to a more basic isoelectric point (pI). It is unfortunate that the IEF image shown in this article has been cut just below the area corresponding to this protein. The subsequent investigations of deglycosylation were very interesting and corroborate our results about this 42-kDa protein. It is unfortunate that they were arbitrarily attributed to the bands shown by IEF.
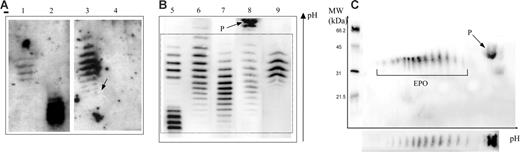
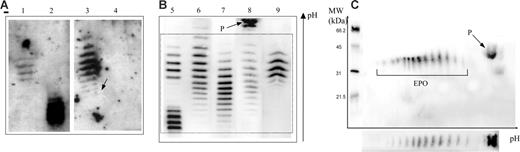
Epo profiles obtained by double-blotting. Panel A is reproduced from Beullens et al.1(Fig1A), and panels B and C are results from our laboratory. (A) Lane 1 shows epoetin β; lane 2, darbepoetin α; lane 3, urine sample considered as “false positive” (the arrow shows a white hole corresponding to ineffective transfer of proteins in this area); and lane 4, the same sample as in lane 3 1 hour later. (B) Lane 5 shows a mixture of epoetin β and darbepoetin α; lane 6, natural urinary Epo from a sample taken after strenuous exercise (note some shift toward the cathode of the banding pattern); lane 7, natural urinary Epo; lane 8, urine sample showing the protein (P) unrelated to Epo outside the window of integration (dotted box) used for interpretation of an antidoping control result; and lane 9, urine sample in case of epoetin administration (note the difference with the natural urinary Epo pattern even in the case of the post-strenuous exercise sample). (C) Two-dimensional electrophoresis of a urine sample showing both Epo and protein P.
Epo profiles obtained by double-blotting. Panel A is reproduced from Beullens et al.1(Fig1A), and panels B and C are results from our laboratory. (A) Lane 1 shows epoetin β; lane 2, darbepoetin α; lane 3, urine sample considered as “false positive” (the arrow shows a white hole corresponding to ineffective transfer of proteins in this area); and lane 4, the same sample as in lane 3 1 hour later. (B) Lane 5 shows a mixture of epoetin β and darbepoetin α; lane 6, natural urinary Epo from a sample taken after strenuous exercise (note some shift toward the cathode of the banding pattern); lane 7, natural urinary Epo; lane 8, urine sample showing the protein (P) unrelated to Epo outside the window of integration (dotted box) used for interpretation of an antidoping control result; and lane 9, urine sample in case of epoetin administration (note the difference with the natural urinary Epo pattern even in the case of the post-strenuous exercise sample). (C) Two-dimensional electrophoresis of a urine sample showing both Epo and protein P.
In summary, the IEF pattern shown by Beullens et al has been interpreted as unrelated to Epo, whereas it corresponded to a bad-quality image (such a result would have been categorically rejected from any interpretation in antidoping control) of a well-known Epo pattern observed after particular conditions of strenuous exercise. The figure enables one to compare the IEF results of Beullens et al1 with well-identified Epo patterns as observed in our laboratory from several hundreds of samples. A 2-dimensional electrophoresis has been introduced to support our statements.
It is disappointing that such poor-quality experiments and misinterpreted results led the authors to believe that the validity of the Epo antidoping test could be questioned.
False-positive detection of rhEpo remains a real concern
Epo test results are clearly not always interpreted identically by all WADA-accredited laboratories. For example, the athlete we examined was initially found guilty of rhEpo abuse by 2 WADA-accredited laboratories. During the ensuing appeal procedure, it was disclosed by a third WADA-accredited laboratory that the original data leading to the suspension of this athlete had been interpreted wrongly by the former 2 WADA laboratories, and that the tests were actually negative for rhEPO.
According to the official WADA identification criteria for rhEPO (Figure 1A), the third lane of our IEF immunoblot2 is positive for rhEpo, and the fourth lane is negative (Figure 1B). We disagree with the interpretation of these data according to other ad-hoc “WADA rules.” Strikingly, lane 6 of Dr Lasne's figure is presented as endogenous Epo but should be interpreted according to the official WADA criteria as rhEpo (Figure 1C). As for the required controls, our IEF data show a negative urine sample as well as samples of purified rhEpo, which served as positive controls (Figure 1B).2 We are surprised by the criticism that we did not detect endogenous Epo, since a WADA report indicates that up to 20% of the analyzed specimens do not contain endogenous Epo.3 The background staining in Figure 1B does not interfere with the application of the Epo identification criteria and, hence, we do not understand why our data should be rejected because they are “poor-quality images.” As a recent Nature editorial puts it: “Slightly dirty images reflect the real world.”4(p892) A WADA report furthermore acknowledges that protein-rich samples cause background staining.3
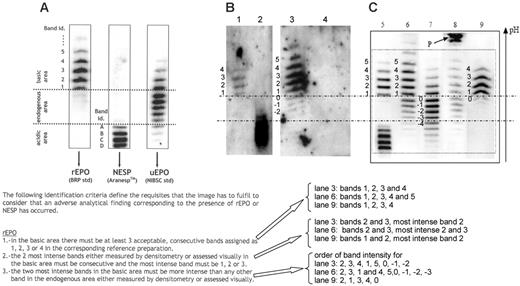
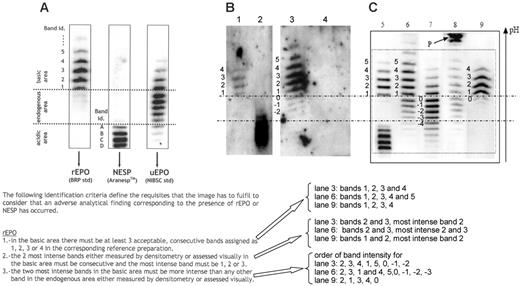
Epo profiles obtained by double immunoblotting. (A) Acceptance criteria for rhEpo, as defined by WADA.1 (B) Figure 1A from Beullens et al.2 Lane 1, epoetin β; lane 2, darbepoietin α; lane 3, “false-positive” urine sample collected immediately after exercise; lane 4, negative urine sample collected 1 hour after the sample in lane 3. (C) Figure 1 from Lasne. Lane 5, mixture of epoetin β and darbepoietin α; lane 6, urine sample after strenuous exercise; lane 7, natural urinary Epo; lane 8, urine sample showing protein P, unrelated to Epo; lane 9, urine sample in case of epoetin administration. Bands in panels B and C are numbered according to the criteria defined in panel A. Using the acceptance criteria of panel A, lanes 3 (panel B), 6, and 9 (panel C) should be considered as an adverse analytical finding corresponding to the presence of rhEpo.
Epo profiles obtained by double immunoblotting. (A) Acceptance criteria for rhEpo, as defined by WADA.1 (B) Figure 1A from Beullens et al.2 Lane 1, epoetin β; lane 2, darbepoietin α; lane 3, “false-positive” urine sample collected immediately after exercise; lane 4, negative urine sample collected 1 hour after the sample in lane 3. (C) Figure 1 from Lasne. Lane 5, mixture of epoetin β and darbepoietin α; lane 6, urine sample after strenuous exercise; lane 7, natural urinary Epo; lane 8, urine sample showing protein P, unrelated to Epo; lane 9, urine sample in case of epoetin administration. Bands in panels B and C are numbered according to the criteria defined in panel A. Using the acceptance criteria of panel A, lanes 3 (panel B), 6, and 9 (panel C) should be considered as an adverse analytical finding corresponding to the presence of rhEpo.
We have used the term “false positive” according to the current terminology in diagnostic medicine in the sense of a type I error (ie, when a test incorrectly reports that it has found a positive result when none really exists). The same terminology has been used in a WADA report.3 Lasne argues that our “false-positive” result stems from endogenous Epo that under particular conditions of strenuous exercise migrates more basically. This is a speculation not supported by any evidence. In fact, a WADA-supported study concluded that strenuous exercise does not affect the Epo pattern in such a way as to require changes in the identification criteria.5 Lasne apparently did not understand the problem generated by the detection of urinary “rhEpo” immediately after exercise but not in a sample that was obtained 1 hour later (Figure 1B). As the half-life of Epo is about 8 hours, this indicates that the detected signals at 0 hours are not derived from Epo itself.
We believe that the lack of specificity of the anti-Epo antibody (clone AE7A5) lies at the heart of the false-positive detection of rhEpo. This issue has also been raised by Khan et al,6 and is not unexpected since this antibody is sold for research use only. We note that Lasne is the first WADA-supported investigator admitting to the nonspecificity of this antibody. It is very surprising that this cross-reactivity has not been noted in the thousands of Epo tests that have been performed so far. Lasne claims that this lack of specificity does not interfere with the Epo test because the interfering signals are from “a” protein that migrates more basically than the Epo isoforms. However, Figure 2 of our study2 shows that the AE7A5 antibody binds to multiple polypeptides during immunoblotting, even at much lower protein concentrations than those used for the Epo test. It is incorrect that this cross-reactivity is only detected after SDS-PAGE. Lane 8 of Lasne's figure proves that this also applies to IEF. Lasne's claim that we attributed the false-positive rhEpo-signals to the 42-kDa cross-reacting band is also incorrect. Any cross-reacting protein(s) can be responsible for this.
We firmly maintain our conclusion that, in the examined athlete, strenuous exercise did result in the false-positive detection of rhEpo. The diagnostic use of an antibody that is not monospecific and the evident use of ad-hoc interpretation criteria by the WADA-accredited laboratories are worrisome and difficult to reconcile with the claim that the rhEpo test is infallible.
Correspondence: Mathieu Bollen, Division of Biochemistry, Department of Molecular Cell Biology, Faculty of Medicine, Catholic University of Leuven, B-3000 Leuven, Belgium; mathieu.bollen@med.kuleuven.be.