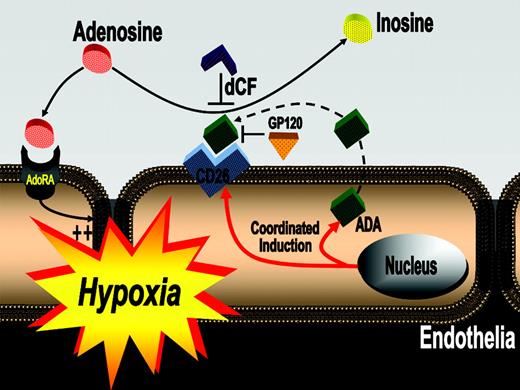
Extracellular levels of adenosine increase during hypoxia. While acute increases in adenosine are important to counterbalance excessive inflammation or vascular leakage, chronically elevated adenosine levels may be toxic. Thus, we reasoned that clearance mechanisms might exist to offset deleterious influences of chronically elevated adenosine. Guided by microarray results revealing induction of endothelial adenosine deaminase (ADA) mRNA in hypoxia, we used in vitro and in vivo models of adenosine signaling, confirming induction of ADA protein and activity. Further studies in human endothelia revealed that ADA-complexing protein CD26 is coordinately induced by hypoxia, effectively localizing ADA activity at the endothelial cell surface. Moreover, ADA surface binding was effectively blocked with glycoprotein 120 (gp120) treatment, a protein known to specifically compete for ADA-CD26 binding. Functional studies of murine hypoxia revealed inhibition of ADA with deoxycoformycin (dCF) enhances protective responses mediated by adenosine (vascular leak and neutrophil accumulation). Analysis of plasma ADA activity in pediatric patients with chronic hypoxia undergoing cardiac surgery demonstrated a 4.1 ± 0.6-fold increase in plasma ADA activity compared with controls. Taken together, these results reveal induction of ADA as innate metabolic adaptation to chronically elevated adenosine levels during hypoxia. In contrast, during acute hypoxia associated with vascular leakage and excessive inflammation, ADA inhibition may serve as therapeutic strategy.
Introduction
Physiologic adaptation and pathophysiologic response to hypoxia are currently areas of intense investigation, and several reports suggest that both transcriptional and metabolic pathways may contribute to a broad range of diseases.1 For example, during episodes of hypoxia/ischemia, polymorphonuclear leukocytes (PMNs) are mobilized from the intravascular space to the interstitium,2,3 and such responses may contribute significantly to tissue damage during subsequent reperfusion.4 Emigration of PMNs through the endothelial barrier is associated with a disruption of tissue barriers, creating the potential for vascular fluid leakage and subsequent edema formation.5,6
At the same time, extracellular nucleotide metabolites (particularly adenosine) may function as endogenous anti-inflammatory mediators during hypoxia.1,3,4,6,7 Vascular adenosine signaling during hypoxia has been implicated, dampening pathophysiologic changes related to increased tissue permeability, accumulation of inflammatory cells, and transcriptional induction of proinflammatory cytokines during hypoxia.1,8 Several lines of evidence support this assertion: first, adenosine receptors are widely expressed on vascular endothelial cells, and have been studied for their capacity to modulate inflammation;1,3,5 second, murine models of inflammation and/or hypoxia provide evidence for adenosine receptor signaling as a mechanism for regulating hypoxia responses in vivo. Indeed, mice genetically deficient in surface enzymes necessary for adenosine generation (ecto-apyrase, CD39 [conversion of ATP to AMP] and ecto-5′-nucleotidase, CD73 [conversion of AMP to adenosine]) show increased hypoxia-associated tissue damage and vascular leak syndrome during hypoxia.5,6 Third, hypoxia accompanies the normal inflammatory response9-11 and is associated with significantly increased levels of adenosine.12,13 The exact source(s) of adenosine are not well defined, but likely include a combination of increased intracellular metabolism and amplified extracellular phosphohydrolysis of adenine nucleotides via surface ectonucleotidases.5,6 In addition, recent studies have also shown that hypoxia inducible factor (HIF)-dependent transcriptional repression of equilibrative nucleoside transporters (ENTs) results in decreased capacity of the vascular endothelium to transport extracellular adenosine, thereby providing an additional mechanism to elevate vascular adenosine levels during hypoxia.14
Despite the central role of adenosine in innate inflammatory responses, chronically increased levels of adenosine may be detrimental.15 For example, levels of adenosine are increased in the lungs of asthmatics,16 and correlate with the degree of inflammatory insult,17 suggesting a provocative role of adenosine in asthma or chronic obstructive pulmonary disease.18 In addition, adenosine deaminase (ADA)-deficient mice develop signs of chronic pulmonary injury in association with elevated pulmonary adenosine levels. ADA-deficient mice, in fact, die within weeks after birth from severe respiratory distress,19 and recent studies suggest that attenuation of adenosine signaling may reverse the severe pulmonary phenotypes in ADA-deficient mice, suggesting that chronic adenosine elevation can affect signaling pathways that mediate aspects of chronic lung disease.20,21
Given the biological necessity to balance the benefits of elevated extracellular adenosine levels with potential chronic toxicity, we sought to determine whether mechanisms exist to degrade extracellular adenosine in models of increased adenosine (hypoxia). As guided by initial microarray analyses, studies in endothelial cells as well as in chronically hypoxic human subjects revealed parallel induction of extracellular ADA and CD26 by hypoxia, thereby increasing the capacity for extracellular adenosine catabolism. Furthermore, in experiments with mice subjected to normobaric hypoxia, the administration of an ADA inhibitor, deoxycoformycin (dCF), ameliorated both pulmonary edema and tissue neutrophil accumulation. Based on these results and on previous work suggesting deleterious effects of chronically elevated adenosine levels,15,19-21 inhibition of ADA may serve as a therapeutic strategy to further enhance adenosine signaling and counterbalance vascular effects of acute hypoxia. In contrast, during chronically elevated adenosine levels, ADA induction may serve as innate adaptation to protect the organism from potentially deleterious effects of long-term elevation of adenosine or its metabolites.
Patients, materials, and methods
Endothelial cell culture
Human microvascular endothelial cells (HMEC-1s) were cultured as described previously.22 In addition, human umbilical vein endothelial cells (HUVECs) were isolated and cultured as described previously.23 HUVECs were used up to 4 passages, then the cells were discarded and new cells were freshly isolated from an umbilical cord.
Analysis of transcript levels
Transcriptional profile of endothelial cells (HMEC-1s) subjected to normobaric hypoxia (24 hours) was compared with that of normoxic control endothelia using quantitative genechip expression arrays (Affymetrix, Santa Clara, CA) as described previously,24 and mRNA was quantified by real-time reverse transcription-polymerase chain reaction (RT-PCR, iCycler; Bio-Rad Laboratories, Hercules, CA), as described previously.6 For ADA (Enzyme Classification [E.C.] no. 3.5.4.4), the primer set consisted of the sense primer 5′-CAC ACG TAT ACC TCG GCA TG-3′ and the antisense primer 5′-GCC ATG GGC TTC TTT ATT GA -3′; for CD26, the primer set consisted of the sense primer 5′-AAA ATG AGT CCA AGG AAG TT -3′ and the antisense primer 5′-AAG CAG CTT GAA ACT GAG-3′. After approval by the Institutional Review Board of the Brigham and Women's Hospital (Boston, MA), real-time RT-PCR was also performed with RNA isolated from human saphenous vein after ex vivo exposure to hypoxia as described previously.6
Immunoblotting experiments
HMEC-1 proteins were analyzed by Western blot (total protein) or by immmunoprecipition of biotinylated membranes (surface protein) as described previously.6 Where indicated, goat polyclonal anti-ADA as described previously,25 or rabbit polyclonal anti-CD26 (Santa Cruz Biotechnology, Santa Cruz, CA) were used.
Measurement of ADA activity in cell-culture experiments
HMEC-1s were grown to full confluency and exposed as indicated to normoxia or different degrees of hypoxia. We first measured adenosine conversion to inosine in HMEC-1 monolayers with different adenosine concentrations (1-500 μM) and found optimal resolution when using 50 μM adenosine. After washing in Hanks balanced salt solution (HBSS), addiing 10 μM dipyridamole, and adjusting the concentration of adenosine in the supernatant to 50 μM, samples were collected at indicated time points, and inosine concentrations were measured by high-performance liquid chromatography (HPLC) with a P680 pump and an UVD 170 detector on a reverse-phase column (Grom-Sil 120-ODS-ST-5 μm; 150 ×3 mm; Grom, Herrenberg, Germany) using a mobile phase gradient (A: 0.01 M KH2PO4 [pH 5]; B: A + 25% acetonitrile [pH 5]; gradient: A to 100% B in 2 minutes). Ultraviolet absorption was measured at 260 nm, with a retention time for inosine of 3.2 and for adenosine of 3.7 min (Figure 2A). In subsets of experiments, ADA secretion into the supernatant was measured. For this purpose, HMEC-1s were exposed to hypoxia over 44 hours, and the cell media was replaced with HBSS supplemented with 10 mM HEPES (pH 7.4). After 4 hours, the supernatant was removed from the cells, its adenosine concentration was adjusted to 50 μM, and inosine generation was measured using the HPLC technique. In subsets of experiments, ADA activity was measured in the lysates of endothelia. For this purpose, HMEC-1s were exposed to normoxia or 48 hours of hypoxia, then lysed with cold H2O, and ADA activity was measured using the HPLC technique after the addition of 1/10 volume of 10 × HBSS + HEPES (pH 7.4). In subsets of experiments, ADA binding to the ADA-complexing protein CD26 was inhibited with HIV-1 glycoprotein 120 (gp120; Protein Science Corporation, Boston, MA). For this purpose, HMEC-1s were exposed to normoxia or hypoxia (48 hours), and washed with 100 nM gp120 in HBSS over 10 minutes at 37°C; ADA activity was measured using the HPLC technique.
Macromolecule paracellular permeability assay
As described previously,6,23 HMEC-1s were grown on polycarbonate permeable supports and studied 7 to 10 days after seeding. In subsets of experiments, bovine ADA (Sigma-Aldrich, St Louis, MO), dCF (pentostatin; Wyeth Pharma, Münster, Germany) and gp120 (Protein Science, Meriden, CT) were used in concentrations as indicated.
In vivo hypoxia model
C57BL/6/129 svj mice were matched according to sex, age, and weight, and were then exposed to normobaric hypoxia (8% O2, 92% N2) or room air for 4 hours (n = 6 animals per condition). Following hypoxia/normoxia exposure, the animals were killed and plasma samples were obtained via cardiac puncture. Plasma ADA activity was measured as described in “Measurement of ADA activity in plasma. In subsets of experiments, total organ vascular permeability was quantified by intravascular administration of Evan blue as described previously following injection with dCF (1 mg/kg intraperitoneally and 1 mg/kg subcutaneously) or PBS (n = 6 animals per condition). This protocol was in accordance with National Institutes of Health (NIH) guidelines for use of live animals and was approved by the Institutional Animal Care and Use Committee at Brigham and Women's Hospital.
Measurement of ADA activity in plasma
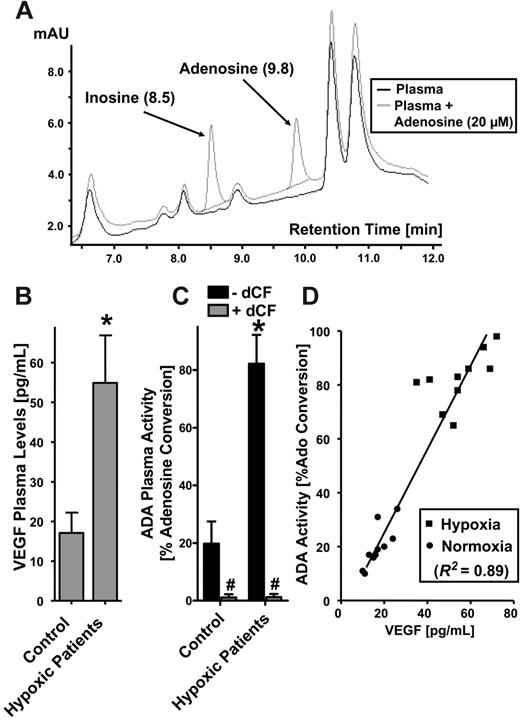
Plasma ADA activity was measured using a murine model of hypoxia and in pediatric patients with known chronic hypoxia. The study was approved by the Institutional Review Board of Children's Hospital (Boston, MA), and written informed parental consent was obtained from each patient. The study population consisted of 10 consecutive patients undergoing a bidirectional Glenn procedure with known oxygen saturation of less than 90%, and 10 age- and sex-matched controls undergoing noncardiac surgery. Plasma samples were obtained prior to the operation after the induction of anesthesia. Blood samples were anticoagulated with EDTA, spun, and the plasma was then isolated and stored at -80°C. In order to measure ADA activity in plasma samples, we first measured adenosine conversion to inosine in previously obtained plasma samples from volunteers using different concentrations of adenosine (1-500 μM) and found optimal resolution of adenosine metabolism to inosine in plasma when using 20 μM adenosine concentrations. These conditions were used throughout for further measurements. To measure ADA plasma activity in the study subjects, 49 μL of plasma were warmed up to 37°C, and 1 μL of a 1 mM adenosine stock solution was added, thus resulting in a starting concentration of 20 μM adenosine. After 30 minutes, a sample was injected into the HPLC, using a P680 pump and an UVD 170 detector on a reverse-phase column (Reprosil-Pur C18-AQ, 5 μm; Maisch, Ammerbuch-Entringen, Germany) with a mobile phase gradient (A: 0.76g NH4COO/l H2O; B: A + 25% Methanol, gradient: 0-3 minutes: 10% B, 3-9 minutes 10%-100% B, 9-13 minutes: 100% B). Ultraviolet absorption was measured at 260 nm, with a retention time for inosine of 8.5 minutes and for adenosine of 9.8 min (Figure 6A). ADA activity was expressed as percent conversion of adenosine to inosine. In control experiments, the ADA inhibitor dCF was added (10 μM) to demonstrate specificity for ADA.
Measurement of VEGF in human or murine plasma
Plasma samples were thawed to 4°C and vascular endothelial growth factor (VEGF) concentrations were measured using a human VEGF quantitative enzyme-linked immunosorbent assay (ELISA) system (R&D Systems, Minneapolis, MN; catalog no. DVE00 for human plasma and MMV00 for murine plasma), according to the instructions given by the manufacturer. All analyses were made in duplicate and mean values were used for statistical analysis.
Data analysis
Data were compared by 2-factor analysis of variance (ANOVA), or by Student t test where appropriate. Values are expressed as the mean ± SD from at least 3 separate experiments.
Results
Endothelial ADA mRNA and protein are induced by hypoxia
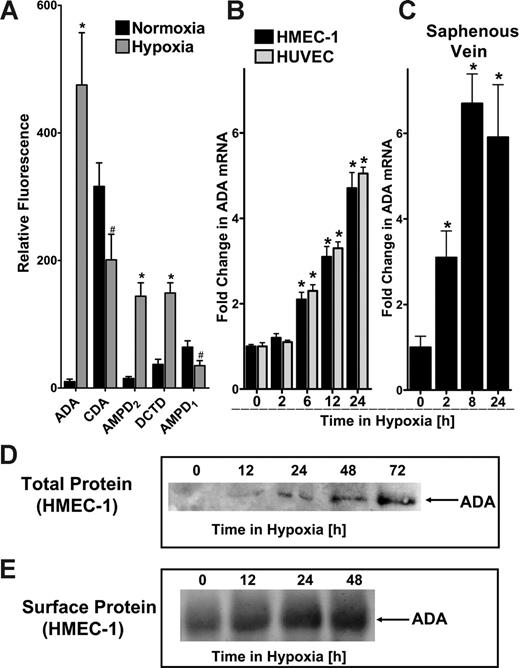
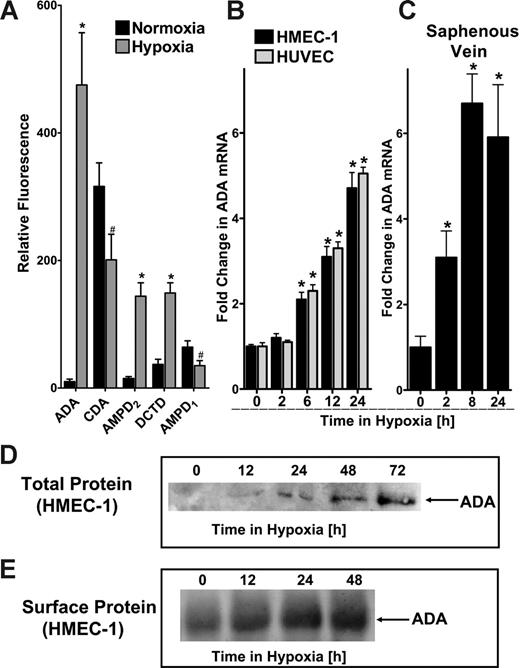
We have previously shown that hypoxia is associated with transcriptionally regulated increases in adenine nucleotide phosphohydrolysis and signaling.5,6,24 Based on these previous studies, we hypothesized that catabolic mechanisms must exist to counter such large increases in adenosine. With this rationale, we pursued identification of potential adenosine catabolic pathways elicited by hypoxia. Initial studies using transcriptional profiling of mRNA derived from hypoxic endothelia (HMEC-1, pO2 20 mm Hg, 24 hours) revealed significant changes in a number of nucleotide and nucleoside deaminase transcripts (Figure 1A). For example, these studies revealed decreased levels of AMP deaminase (AMPD1, 55% ± 9.1% decrease), and cytidine deaminase (CDA, 64% ± 11.2% decrease). By contrast, myoadenylate deaminase (AMPD2) and deoxycytidine deaminase (DCTD) were induced (9 ± 1.1-fold and 4 ± 0.3-fold, respectively). Most dramatically, these analyses identified a 47 ± 8.8-fold increase in ADA mRNA by hypoxia. Given the magnitude of this latter response, we pursued biochemical and functional aspects of ADA induction by hypoxia.
Comparison of HMEC-1 and HUVEC mRNA levels by real-time PCR confirmed our initial microarray findings that ADA mRNA is prominently induced by hypoxia (Figure 1B). Similar results of ADA inducibility were found when human saphenous vein tissues were subjected to hypoxia, indicating that such findings are not limited to cultured endothelia, and likely occur at the whole-tissue level (Figure 1C). We extended these findings to examine ADA protein levels in hypoxia. Western blot analysis of total ADA protein revealed a time-dependent induction of ADA (range, 12-72 hours). Based on previous reports that ADA can be localized to the cell surface via interaction with CD26,26 we considered the possibility that such increases in ADA protein levels may be localized to the cell surface. As shown in Figure 1E, immunoprecipitation of ADA from surface-biotinylated protein revealed a time-dependent induction of surface ADA. Taken together, these results demonstrate that ADA is rapidly induced by hypoxia, and such increases of ADA protein are demonstrable at the cell surface.
ADA is rapidly induced by hypoxia. (A) Microarray analysis of individual nucleoside/nucleotide deaminases in response to hypoxia (ADA, CDA, AMPD2, DCTD, and AMPD1). Confluent HMEC-1s were exposed to normoxia (pO2 147 mm Hg, 18 hours) or hypoxia (24-hour exposure to pO2 20 mm Hg) and the relative expression of individual deaminases was quantified from total RNA by microarray analysis. *Increased fluorescence, P < .01. #Decreased fluorescence, P < .01. Note the dramatic induction of ADA with hypoxia (47.5-fold). (B) Confluent HMEC-1 or HUVEC monolayers were exposed to normoxia (pO2 147 mm Hg, 18 hours) or hypoxia (pO2 20 mm Hg) as indicated. Total RNA was isolated, and ADA mRNA levels were determined by real-time RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from 3 experiments in each condition (*P < .01). (C) Human saphenous veins were obtained from patients undergoing aorto-coronary bypass surgery and exposed ex vivo to ambient normoxia (pO2 147 mm Hg, 24 hours) or hypoxia (pO2 20 mm Hg for 2, 8, or 24 hours). After total RNA isolation, real-time PCR was performed to investigate ADA inducibility by hypoxia. Data were calculated relative to an internal control (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from 3 experiments in each condition (*P < .01). (D) Increase in total ADA protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia (0-72 hours), washed, and lysed. Lysates were resolved by SDS-PAGE, and resultant Western blots were probed with a polyclonal goat antibody directed against human ADA. A representative experiment of 3 is shown. (E) Increase in surface ADA protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia (0-48 hours), monolayers were washed, surface proteins were biotinylated, and cells were lysed. ADA was immunoprecipitated with a polyclonal goat antibody directed against human ADA. Immunoprecipitates were resolved by SDS-PAGE, and resultant Western blots were probed with avidin-peroxidase. A representative experiment of 3 is shown.
ADA is rapidly induced by hypoxia. (A) Microarray analysis of individual nucleoside/nucleotide deaminases in response to hypoxia (ADA, CDA, AMPD2, DCTD, and AMPD1). Confluent HMEC-1s were exposed to normoxia (pO2 147 mm Hg, 18 hours) or hypoxia (24-hour exposure to pO2 20 mm Hg) and the relative expression of individual deaminases was quantified from total RNA by microarray analysis. *Increased fluorescence, P < .01. #Decreased fluorescence, P < .01. Note the dramatic induction of ADA with hypoxia (47.5-fold). (B) Confluent HMEC-1 or HUVEC monolayers were exposed to normoxia (pO2 147 mm Hg, 18 hours) or hypoxia (pO2 20 mm Hg) as indicated. Total RNA was isolated, and ADA mRNA levels were determined by real-time RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from 3 experiments in each condition (*P < .01). (C) Human saphenous veins were obtained from patients undergoing aorto-coronary bypass surgery and exposed ex vivo to ambient normoxia (pO2 147 mm Hg, 24 hours) or hypoxia (pO2 20 mm Hg for 2, 8, or 24 hours). After total RNA isolation, real-time PCR was performed to investigate ADA inducibility by hypoxia. Data were calculated relative to an internal control (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from 3 experiments in each condition (*P < .01). (D) Increase in total ADA protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia (0-72 hours), washed, and lysed. Lysates were resolved by SDS-PAGE, and resultant Western blots were probed with a polyclonal goat antibody directed against human ADA. A representative experiment of 3 is shown. (E) Increase in surface ADA protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia (0-48 hours), monolayers were washed, surface proteins were biotinylated, and cells were lysed. ADA was immunoprecipitated with a polyclonal goat antibody directed against human ADA. Immunoprecipitates were resolved by SDS-PAGE, and resultant Western blots were probed with avidin-peroxidase. A representative experiment of 3 is shown.
Hypoxia-induced ADA is enzymatically active
We next determined whether hypoxia-inducible ADA was enzymatically active. To measure ADA enzyme activity on the endothelial surface, we exposed endothelia over different time periods to hypoxia (pO2 20 mm Hg, exposure time of 12 to 72 hours). Following hypoxia exposure, the time-dependent generation of inosine from exogenous adenosine (50 μM final concentration) was used to determine ADA activity (see HPLC tracing, Figure 2A). As shown in Figure 2B, measurements of total ADA activity from hypotonic lysates of endothelia revealed a hypoxia-dependent increase of total ADA activity, with maximal increases of 7.1 ± 0.52-fold over that of normoxia controls (P < .01). Such activity was more than 95% inhibited by the addition of dCF (Figure 2B). Given our findings of ADA protein localization at the cell surface (Figure 1E), we next determined whether hypoxia-dependent increases of cell-surface ADA enzyme activity were evident on intact endothelial cells. To do this, we examined the time-dependent generation of inosine from exogenous adenosine (50 μM final concentration) in the presence of dipyridamole (100 nM), a condition previously shown to potently inhibit adenosine uptake.14 Cell-surface ADA activity was increased in a hypoxia time-dependent fashion with maximal induction after 48 hours of hypoxia exposure (5.0 ± 0.61-fold over that of normoxia; P < .01 by ANOVA). Such surface ADA activity was inhibited more than 95% by dCF (Figure 2C).
ADA induced by hypoxia is enzymatically active. (A) To measure ADA enzyme activity on the endothelial surface, HMEC-1s were exposed over indicated time periods to hypoxia (pO2 20 mm Hg), 50 μM adenosine was added to the supernatant (HBSS) of intact HMEC-1s, and inosine generation was measured via HPLC. The HPLC tracing obtained from the supernatant is displayed and retention times for inosine (3.2 min) and for adenosine (3.7 min) are indicated (black line indicates HBSS alone; gray line, HBSS after addition of adenosine [50 μM]). (B) Induction of ADA activity in total-cell lysates of endothelia by hypoxia. To obtain an estimate of total ADA activity increase with hypoxia, the ADA activity in lysates of HMEC-1s was measured. (C) Induced, enzymatically active ADA is localized to the cell surface. ADA enzyme activity was measured in intact HMEC-1s by adding 50 μM adenosine to the supernatant (HBSS) and measuring inosine generation. All experiments were performed in the presence of 10 μM dipyridamole to prevent endothelial adenosine uptake. To confirm that the observed increase in inosine generation with hypoxia exposure reflects ADA activity, controls with 100 nM dCF were performed in normoxic (dCF [Norm]) or posthypoxic (dCF [Hyp 48 h]) endothelia. Note the robust hypoxia induction of functional ADA to the cell surface. (D) ADA release into the supernatant is increased with hypoxia. To measure ADA release into the supernatant, HMEC-1s were exposed to normoxia or hypoxia (HMEC-1, pO2 20 mm Hg, 48 hours). During the last 4 hours, the media was replaced with HBSS and ADA activity in the cell supernatant was measured. In controls, dCF (dCF [Hyp 48 h], 100 nM) inhibited soluble ADA activity in posthypoxic supernatants. In contrast, the HIV-1 envelope glycoprotein gp120 (gp120 [Hyp 48 h], 100 nM) did not affect ADA activity in the supernatant. Error bars indicate SD.
ADA induced by hypoxia is enzymatically active. (A) To measure ADA enzyme activity on the endothelial surface, HMEC-1s were exposed over indicated time periods to hypoxia (pO2 20 mm Hg), 50 μM adenosine was added to the supernatant (HBSS) of intact HMEC-1s, and inosine generation was measured via HPLC. The HPLC tracing obtained from the supernatant is displayed and retention times for inosine (3.2 min) and for adenosine (3.7 min) are indicated (black line indicates HBSS alone; gray line, HBSS after addition of adenosine [50 μM]). (B) Induction of ADA activity in total-cell lysates of endothelia by hypoxia. To obtain an estimate of total ADA activity increase with hypoxia, the ADA activity in lysates of HMEC-1s was measured. (C) Induced, enzymatically active ADA is localized to the cell surface. ADA enzyme activity was measured in intact HMEC-1s by adding 50 μM adenosine to the supernatant (HBSS) and measuring inosine generation. All experiments were performed in the presence of 10 μM dipyridamole to prevent endothelial adenosine uptake. To confirm that the observed increase in inosine generation with hypoxia exposure reflects ADA activity, controls with 100 nM dCF were performed in normoxic (dCF [Norm]) or posthypoxic (dCF [Hyp 48 h]) endothelia. Note the robust hypoxia induction of functional ADA to the cell surface. (D) ADA release into the supernatant is increased with hypoxia. To measure ADA release into the supernatant, HMEC-1s were exposed to normoxia or hypoxia (HMEC-1, pO2 20 mm Hg, 48 hours). During the last 4 hours, the media was replaced with HBSS and ADA activity in the cell supernatant was measured. In controls, dCF (dCF [Hyp 48 h], 100 nM) inhibited soluble ADA activity in posthypoxic supernatants. In contrast, the HIV-1 envelope glycoprotein gp120 (gp120 [Hyp 48 h], 100 nM) did not affect ADA activity in the supernatant. Error bars indicate SD.
Based on previous reports indicating that ADA may be released into the extracellular milieu,27,28 we determined whether soluble supernatants contain measurable ADA activity, and whether hypoxia might increase soluble ADA activity. To do this, HMEC-1s were subjected to hypoxia (pO2 20 mm Hg, 44 hours), after which media was replaced with hypoxic pre-equlibrated HBSS. After an additional 4 hours of hypoxia, cell-free supernatants were harvested and ADA activity was determined. Soluble ADA activity increased 2.3 ± 0.11-fold relative to normoxia controls (P < .025). Like that at the cell surface, soluble ADA activity was inhibited by more than 95% with the addition of dCF. In contrast, addition of the HIV-1 envelope glycoprotein gp120 (100 nM) did not alter ADA activity (Figure 2D). Taken together, this analysis of functional ADA activity confirms our findings of increased ADA mRNA and protein expression.
Hypoxia coordinates induction of mRNA for the ADA-complexing protein CD26
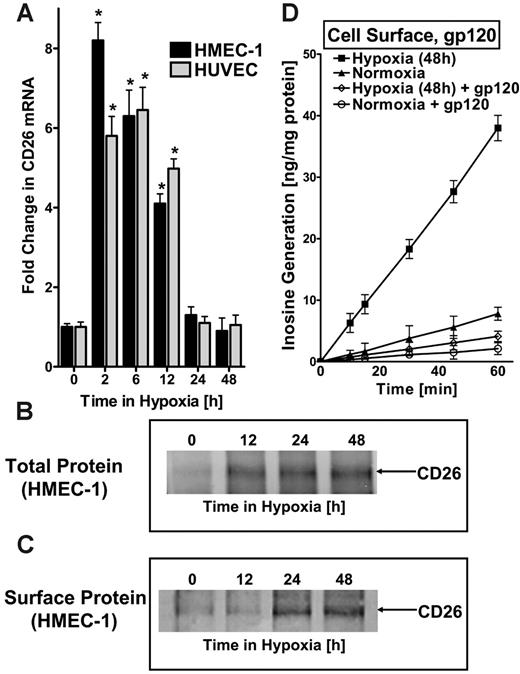
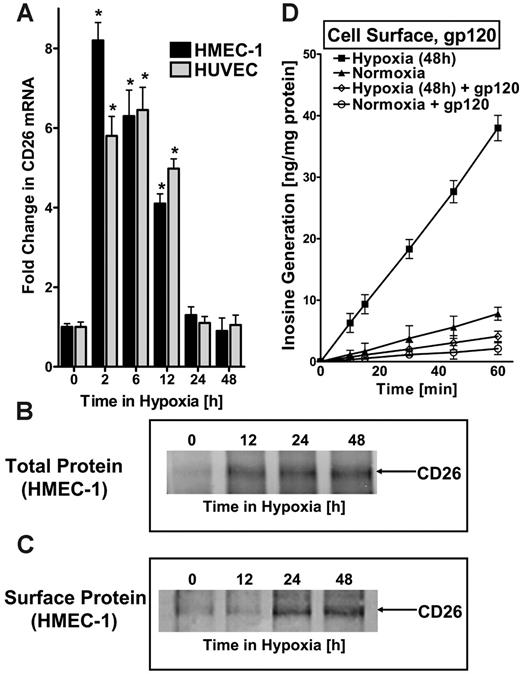
There is no evidence that ADA can be expressed as an integral membrane protein. Therefore, given our findings of increased surface and soluble ADA activity (Figure 2C-D), we wondered whether ADA might be complexed at the cell surface. Significant evidence suggests that surface ADA activity can be associated with the dipeptidyl peptidase CD26.29 Here, we considered the possibility that expression of CD26 may parallel the ADA induction profile observed in hypoxia. To do this, we assessed CD26 expression in HMEC-1s and HUVECs following exposure to hypoxia. This analysis revealed an early and prominent induction, with maximal expression of 8.2 ± 1.1-fold and 5.8 ± 1.2-fold induction in HMEC-1s and HUVECs, respectively, after 2 hours of exposure to hypoxia (Figure 3A), even earlier than ADA. Western blot analysis of total cellular protein and immunoprecipitation of biotinylated surface proteins confirmed CD26 induction by hypoxia and demonstrated its localization to the cell membrane (Figure 3B-C).
To confirm that the observed increase in functional cell-surface ADA reflects hypoxia-induced CD26, we inhibited the interaction of ADA with CD26 using the HIV-1 envelope glycoprotein gp120.30-32 For these purposes, we examined cell-surface ADA activity (see Figure 2C) in the presence of 100 nM gp120, a concentration previously shown to effectively block ADA interaction with CD26.31 As depicted in Figure 3D, the observed induction of cell-surface ADA enzyme activity with hypoxia was nearly completely abolished by gp120 (P < .01). Moreover, gp120 treatment also significantly reduced surface ADA activity of control normoxic endothelia (P < .025). Controls incorporating equivalent amounts of nonspecific protein (bovine serum albumin [BSA]) did not influence surface ADA activity (data not included). In addition, gp120 did not affect soluble ADA activity (Figure 2D). Taken together, these studies indicate that ADA induced by hypoxia is enzymatically active and localized to the cell surface via its counterligand CD26.These studies also suggest a coordinated induction of ADA and the ADA-complexing protein CD26 by hypoxia.
ADA-complexing protein CD26 is coordinately induced by hypoxia. (A) Real-time PCR was used to confirm induction of CD26 mRNA by hypoxia in cultured endothelial cells (HMEC-1s and HUVECs). Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from 3 experiments in each condition (*P < .01). (B) Increase in total CD26 protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia, washed, and lysed. Lysates were resolved by SDS-PAGE, and resultant Western blots were probed with mAb directed against human CD26. A representative experiment of 3 is shown. (C) Increase in surface CD26 protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia, monolayers were washed, surface proteins were biotinylated, and cells were lysed. CD26 was immunoprecipitated with mAb directed against human CD26. Immunoprecipitates were resolved by SDS-PAGE, and resultant Western blots were probed with avidin-peroxidase. A representative experiment of 3 is shown. (D) ADA surface induction requires interaction with CD26. To confirm that the observed increase in enzymatically active cell-surface ADA is bound to hypoxia induced CD26, HIV-1 gp120, a specific inhibitor of ADA interaction with CD26, was used. Postnormoxic or posthypoxic (pO2 20 mm Hg, 48 hours) HMEC-1s were incubated for 10 minutes with 100 nM gp120 in HBSS at 37°C and washed; ADA activity was measured. Note the reduced ADA activity after gp120 treatment in postnormoxic and posthypoxic endothelia (P < .01 by ANOVA). (A, D) Error bars indicate SD.
ADA-complexing protein CD26 is coordinately induced by hypoxia. (A) Real-time PCR was used to confirm induction of CD26 mRNA by hypoxia in cultured endothelial cells (HMEC-1s and HUVECs). Data were calculated relative to internal housekeeping gene (β-actin) and are expressed as fold increase over normoxia ± SD at each indicated time. Results are derived from 3 experiments in each condition (*P < .01). (B) Increase in total CD26 protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia, washed, and lysed. Lysates were resolved by SDS-PAGE, and resultant Western blots were probed with mAb directed against human CD26. A representative experiment of 3 is shown. (C) Increase in surface CD26 protein with hypoxic exposure. Confluent HMEC-1 monolayers were exposed to indicated periods of hypoxia, monolayers were washed, surface proteins were biotinylated, and cells were lysed. CD26 was immunoprecipitated with mAb directed against human CD26. Immunoprecipitates were resolved by SDS-PAGE, and resultant Western blots were probed with avidin-peroxidase. A representative experiment of 3 is shown. (D) ADA surface induction requires interaction with CD26. To confirm that the observed increase in enzymatically active cell-surface ADA is bound to hypoxia induced CD26, HIV-1 gp120, a specific inhibitor of ADA interaction with CD26, was used. Postnormoxic or posthypoxic (pO2 20 mm Hg, 48 hours) HMEC-1s were incubated for 10 minutes with 100 nM gp120 in HBSS at 37°C and washed; ADA activity was measured. Note the reduced ADA activity after gp120 treatment in postnormoxic and posthypoxic endothelia (P < .01 by ANOVA). (A, D) Error bars indicate SD.
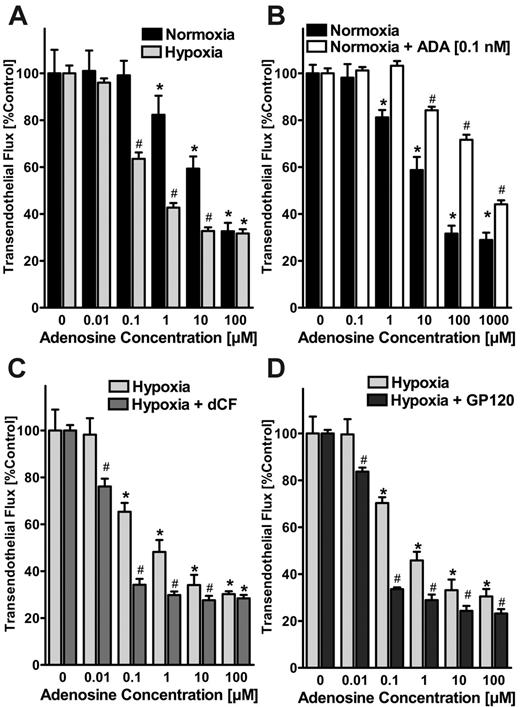
Increased ADA activity dampens adenosine-elicited barrier responses of the endothelium in vitro
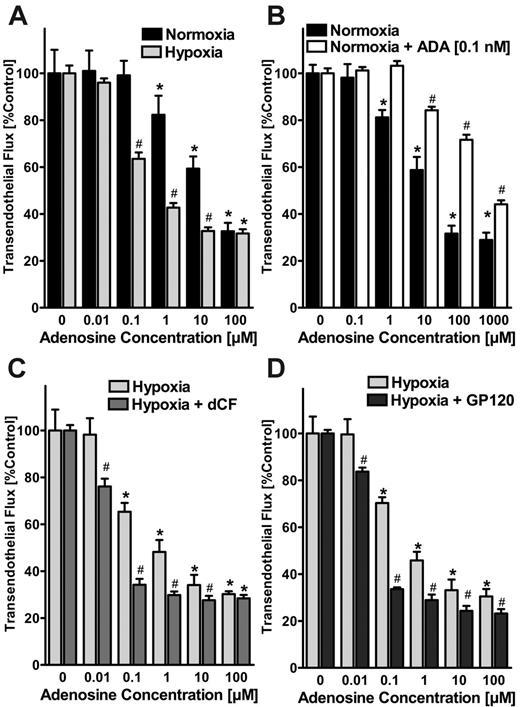
After having shown that endothelial ADA induction is functional and localized to the endothelial surface, we next determined whether such changes in ADA activity might affect signaling mediated by extracellular adenosine. To investigate this possibility, we first compared the effects of adenosine treatment on endothelial barrier function of normoxic and posthypoxic endothelia using a previously described in vitro model.5,6,23 As previously reported,6 we observed that adenosine enhanced barrier function (ie, inhibited the flux of 70-kDa FITC-dextran) to a higher extent in posthypoxic HMEC-1s (pO2 20 mm Hg, 48 hours) than in normoxic HMEC-1s (effective concentration at 50% [EC50] of approximately 1 μM and approximately 30 μM adenosine for normoxia and posthypoxia, respectively; Figure 4A). To investigate the effect of extracellular ADA on paracellular permeability, we first measured ADA concentrations in the supernatant of posthypoxic endothelia by comparing dCF-inhibited inosine generation with known solutions of bovine ADA. In fact, ADA concentrations in supernatants of posthypoxic HMEC-1s (pO2 20 mm Hg, 48 hours) were approximately 1, 25, 50, and 60 pM with 2, 4, 8, and 12 hours of exposure time (data not shown). Based on these findings, we examined the influence of 1-, 10-, and 100-pM concentrations of ADA on paracellular adenosine responses in normoxic endothelia. As shown in Figure 4B, adenosine-induced enhancement of the endothelial barrier function was significantly decreased in the presence of 0.1 nM ADA (estimated EC50, approximately 700 μM). Similarly, when using lower concentrations of ADA, the EC50 of adenosine was increased from approximately 30 μM to approximately 50 and approximately 100 μM with 1- and 10-pM ADA concentrations, respectively (data not shown).
Effects of ADA activity on endothelial adenosine signaling in vitro. (A) Modulation of adenosine signaling by hypoxia. Indicated concentrations of adenosine in HBSS were added to the apical surface of confluent normoxic (48-hour exposure to pO2 147) or posthypoxic (48-hour exposure to pO2 20 mm Hg) HMEC-1s and permeability to FITC-dextran (70 kDa) were quantified. Transendothelial flux was calculated by linear regression (3 samples over 60 minutes) and normalized as a percentage of control (HBSS). Data are derived from 6 monolayers in each condition. *Significant differences from baseline (P < .05). #Differences from baseline and from normoxia (P < .05). (B) Effect of extracellular ADA on paracellular permeability. Measurement of adenosine elicited barrier responses in normoxic endothelia (HMEC-1) with or without the addition of 0.1 nM bovine ADA. Note the dramatic decrease in adenosine-induced enhancement of endothelial barrier function in the presence of 0.1 nM ADA. *Significant differences from baseline (P < .05). #Differences from baseline and untreated controls (P < .05). (C) Effect of ADA inhibitor dCF on adenosine-elicited barrier responses. Posthypoxic endothelia (HMEC-1, pO2 20 mm Hg, 48 hours) were assessed for adenosine-elicited barrier responses in the presence of the highly specific ADA inhibitor dCF (100 nM). Note the dramatically increased adenosine elicited barrier responses with 100 nM dCF. *Significant differences from baseline (P < .05). #Differences from baseline and from untreated controls (P < .05). (D) Effect of inhibiting ADA binding to the ADA-complexing protein CD26 with gp120. Posthypoxic endothelia were washed with 100 nM gp120 in HBSS prior to measuring adenosine-elicited barrier function. Note the increased adenosine elicited barrier responses after gp120 treatment. *Significant differences from baseline (P < .05). #Differences from baseline and from untreated controls (P < .05). (A-D) Data are expressed as mean ± SD of percent control flux with HBSS only.
Effects of ADA activity on endothelial adenosine signaling in vitro. (A) Modulation of adenosine signaling by hypoxia. Indicated concentrations of adenosine in HBSS were added to the apical surface of confluent normoxic (48-hour exposure to pO2 147) or posthypoxic (48-hour exposure to pO2 20 mm Hg) HMEC-1s and permeability to FITC-dextran (70 kDa) were quantified. Transendothelial flux was calculated by linear regression (3 samples over 60 minutes) and normalized as a percentage of control (HBSS). Data are derived from 6 monolayers in each condition. *Significant differences from baseline (P < .05). #Differences from baseline and from normoxia (P < .05). (B) Effect of extracellular ADA on paracellular permeability. Measurement of adenosine elicited barrier responses in normoxic endothelia (HMEC-1) with or without the addition of 0.1 nM bovine ADA. Note the dramatic decrease in adenosine-induced enhancement of endothelial barrier function in the presence of 0.1 nM ADA. *Significant differences from baseline (P < .05). #Differences from baseline and untreated controls (P < .05). (C) Effect of ADA inhibitor dCF on adenosine-elicited barrier responses. Posthypoxic endothelia (HMEC-1, pO2 20 mm Hg, 48 hours) were assessed for adenosine-elicited barrier responses in the presence of the highly specific ADA inhibitor dCF (100 nM). Note the dramatically increased adenosine elicited barrier responses with 100 nM dCF. *Significant differences from baseline (P < .05). #Differences from baseline and from untreated controls (P < .05). (D) Effect of inhibiting ADA binding to the ADA-complexing protein CD26 with gp120. Posthypoxic endothelia were washed with 100 nM gp120 in HBSS prior to measuring adenosine-elicited barrier function. Note the increased adenosine elicited barrier responses after gp120 treatment. *Significant differences from baseline (P < .05). #Differences from baseline and from untreated controls (P < .05). (A-D) Data are expressed as mean ± SD of percent control flux with HBSS only.
As a next step, we measured adenosine-elicited barrier responses in posthypoxic endothelia (HMEC-1, pO2 20 mm Hg, 48 hours) in the presence of the highly specific ADA inhibitor dCF. As shown in Figure 4C, adenosine-elicited barrier responses were significantly increased in the presence of 100 nM dCF (EC50 of approximately 1 μM and approximately 0.05 μM adenosine without and with dCF, respectively; Figure 4C). Similarly, the addition of gp120 (100 nM) resulted in a leftward shift of the dose-response curve to adenosine, suggesting a role of CD26 binding of ADA in regulating vascular adenosine responses (Figure 4D). These studies suggest that extracellular ADA, at levels observed with exposure to hypoxia, significantly inhibits the ability of adenosine to regulate endothelial barrier function.
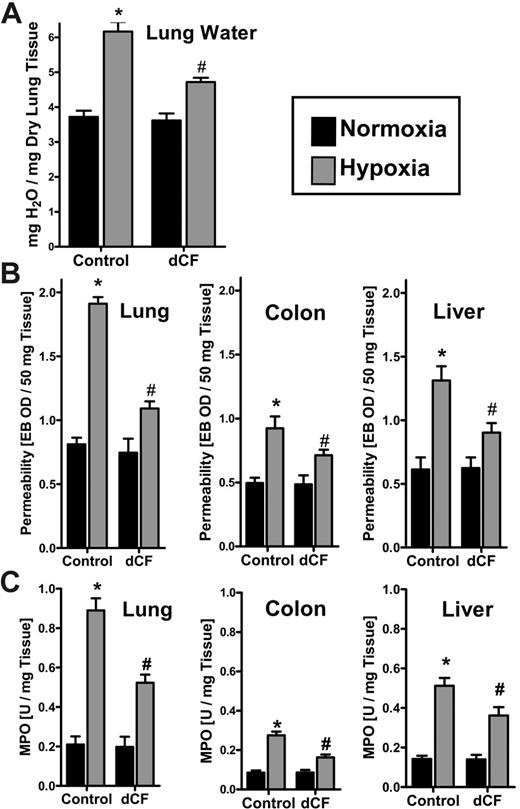
Inhibition of ADA during hypoxia attenuates vascular leak syndrome and PMN tissue accumulation in vivo
We have previously shown that hypoxia promotes vascular leak and tissue PMN accumulation in murine models.5,14 Prompted by these in vitro findings of attenuated adenosine-elicited barrier responses with increased ADA activity, and previous studies suggesting an improvement of endothelial function and decreased vascular leakage during sepsis with ADA inhibition,33-35 we pursued ADA as a pharmacologic target in a previously described in vivo murine model of acute hypoxia. As a first step, we administered the ADA inhibitor dCF (1 mg/kg intraperitoneally and 1 mg/kg subcutaneously) or PBS and subjected animals to either normoxia or normobaric hypoxia (8% O2 and 92% N2) for 4 hours. As the first step, we assessed pulmonary edema by determining lung water content (wet-dry ratio). As shown in Figure 5A, hypoxia increased pulmonary water content by 61% ± 8.9% (P < .025). Such acute increases in lung water were significantly decreased (51% ± 6.3%decrease; P < .01) by administration of dCF, thereby suggesting that endogenously generated adenosine could improve barrier function if it were not degraded by hypoxia-induced ADA.
We next administered the albumin marker Evan blue dye prior to hypoxia. dCF significantly protected animals from hypoxia-induced vascular leak (Figure 5B). Such protective influences of dCF were observed in all organs examined, and were particularly evident in the lung. After demonstrating that inhibition of ADA enzyme activity is associated with decreased vascular leakage, we hypothesized that a reduction of adenosine metabolism at the vascular surface may also function as an anti-inflammatory response. To test this hypothesis, we screened multiple mucosal organs (lung, colon, liver) for PMN accumulation following administration of dCF (Figure 5C). In fact, the PMN marker MPO significantly increased after hypoxia exposure in all animals, but to a lesser extent in all organs of dCF-treated animals compared with PBS controls (P < .05 for all organs). These in vivo findings support the hypothesis that adenosine metabolism via ADA is a critical control point in the regulation of both vascular leak and PMN accumulation within hypoxic tissues, and suggest that inhibition of ADA might represent a therapeutic target in the treatment of vascular leak syndrome and excessive inflammatory cell accumulation in the acute phase of hypoxia.
ADA plasma activity is increased in hypoxic patients
Based on these findings in vitro and in vivo, we extended this analysis to human subjects. Here, we hypothesized that plasma ADA levels should increase in patients with chronic hypoxia. For these purposes, we modified our HPLC-based ADA activity method for measurement in plasma samples. Initial pilot studies with varying adenosine concentrations (range, 1-500 μM) revealed optimal conditions to measure plasma ADA activity using 20 μM exogenous adenosine (Figure 6A). We then verified this method using plasma derived from our murine hypoxia model (8% oxygen for 4 hours).5 Results from this analysis revealed that ADA activity increased by 2.9 ± 0.51-fold in hypoxic animals (P < .01; data not shown). As a control, parallel analyses showed that plasma levels of the established hypoxia marker VEGF36 increased 2.6 ± 0.31-fold in hypoxic animals (P < .01; data not shown). These results verify our methodology and provide a rationale for examining these parameters in human-derived material.
Influence of dCF on pulmonary edema, vascular permeability, and PMN accumulation in vivo. BL/6/129 mice were given injections of dCF (1 mg/kg intraperitoneally and 1 mg/kg subcutaneously) or PBS, and exposed to normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours. (A) Assessment of lung water content in normoxia (▪) and hypoxia ( ) after dCF or PBS treatment. Data are expressed as mean ± SD mg H2O/mg dry tissue, and are pooled from 6 animals per condition. *Significantly different between hypoxia and normoxia (P < .025). #Significantly different between dCF treatment and vehicle control (P < .025). (B) To assess vascular barrier function, animals were administered intravenous Evan blue dye solution (0.2 mL of 0.5% in PBS) prior to normoxia/hypoxia exposure. Animals were killed, and the lung, colon, and liver were harvested. Organ Evan blue concentrations were quantified following formamide extraction (55°C for 2 hours) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Data are expressed as mean ± SD Evan blue optical density (OD)/50mg wet tissue, and are pooled from 6 animals per condition. Note that Evan blue retention increases with hypoxia and decreases with dCF treatment. *Significant differences between normoxia/hypoxia exposure (P < .01). #Significant differences between dCF/PBS treatment groups (P < .025). (C) Organ assessment of PMN accumulation by myeloperoxidase (MPO) measurements in the indicated organs after 4 hours of normoxia/hypoxia exposure (*P < .01 compared with hypoxia; #P < .025 compared with vehicle control). Error bars indicate SD.
) after dCF or PBS treatment. Data are expressed as mean ± SD mg H2O/mg dry tissue, and are pooled from 6 animals per condition. *Significantly different between hypoxia and normoxia (P < .025). #Significantly different between dCF treatment and vehicle control (P < .025). (B) To assess vascular barrier function, animals were administered intravenous Evan blue dye solution (0.2 mL of 0.5% in PBS) prior to normoxia/hypoxia exposure. Animals were killed, and the lung, colon, and liver were harvested. Organ Evan blue concentrations were quantified following formamide extraction (55°C for 2 hours) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Data are expressed as mean ± SD Evan blue optical density (OD)/50mg wet tissue, and are pooled from 6 animals per condition. Note that Evan blue retention increases with hypoxia and decreases with dCF treatment. *Significant differences between normoxia/hypoxia exposure (P < .01). #Significant differences between dCF/PBS treatment groups (P < .025). (C) Organ assessment of PMN accumulation by myeloperoxidase (MPO) measurements in the indicated organs after 4 hours of normoxia/hypoxia exposure (*P < .01 compared with hypoxia; #P < .025 compared with vehicle control). Error bars indicate SD.
Influence of dCF on pulmonary edema, vascular permeability, and PMN accumulation in vivo. BL/6/129 mice were given injections of dCF (1 mg/kg intraperitoneally and 1 mg/kg subcutaneously) or PBS, and exposed to normoxia (room air) or normobaric hypoxia (8% O2 and 92% N2) for 4 hours. (A) Assessment of lung water content in normoxia (▪) and hypoxia ( ) after dCF or PBS treatment. Data are expressed as mean ± SD mg H2O/mg dry tissue, and are pooled from 6 animals per condition. *Significantly different between hypoxia and normoxia (P < .025). #Significantly different between dCF treatment and vehicle control (P < .025). (B) To assess vascular barrier function, animals were administered intravenous Evan blue dye solution (0.2 mL of 0.5% in PBS) prior to normoxia/hypoxia exposure. Animals were killed, and the lung, colon, and liver were harvested. Organ Evan blue concentrations were quantified following formamide extraction (55°C for 2 hours) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Data are expressed as mean ± SD Evan blue optical density (OD)/50mg wet tissue, and are pooled from 6 animals per condition. Note that Evan blue retention increases with hypoxia and decreases with dCF treatment. *Significant differences between normoxia/hypoxia exposure (P < .01). #Significant differences between dCF/PBS treatment groups (P < .025). (C) Organ assessment of PMN accumulation by myeloperoxidase (MPO) measurements in the indicated organs after 4 hours of normoxia/hypoxia exposure (*P < .01 compared with hypoxia; #P < .025 compared with vehicle control). Error bars indicate SD.
) after dCF or PBS treatment. Data are expressed as mean ± SD mg H2O/mg dry tissue, and are pooled from 6 animals per condition. *Significantly different between hypoxia and normoxia (P < .025). #Significantly different between dCF treatment and vehicle control (P < .025). (B) To assess vascular barrier function, animals were administered intravenous Evan blue dye solution (0.2 mL of 0.5% in PBS) prior to normoxia/hypoxia exposure. Animals were killed, and the lung, colon, and liver were harvested. Organ Evan blue concentrations were quantified following formamide extraction (55°C for 2 hours) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Data are expressed as mean ± SD Evan blue optical density (OD)/50mg wet tissue, and are pooled from 6 animals per condition. Note that Evan blue retention increases with hypoxia and decreases with dCF treatment. *Significant differences between normoxia/hypoxia exposure (P < .01). #Significant differences between dCF/PBS treatment groups (P < .025). (C) Organ assessment of PMN accumulation by myeloperoxidase (MPO) measurements in the indicated organs after 4 hours of normoxia/hypoxia exposure (*P < .01 compared with hypoxia; #P < .025 compared with vehicle control). Error bars indicate SD.
Plasma ADA enzyme activity is increased in patients with hypoxia. (A) To develop a technique for measuring ADA activity in plasma, different concentrations of adenosine were added to the plasma and assessed for dCF-inhibited inosine generation. Optimal resolution was found with using 20 μM adenosine (black line indicates plasma alone; gray line, plasma after addition of 20 μM adenosine). (B-C) VEGF and ADA are increased in the plasma of patients with hypoxia. Plasma samples were obtained from 10 pediatric patients with chronic hypoxia (mean oxygen saturation of 82%) undergoing cardiac surgery (bidirectional Glenn procedure) and 10 age- and sex-matched controls (mean oxygen generation of 96%) undergoing noncardiac surgery. Plasma VEGF was measured with standard ELISA technique. ADA activity was measured via HPLC as percent adenosine conversion to inosine. *Significant differences with hypoxia (P < .01). #Differences with dCF treatment (100 nM; P < .001). (B-C) Error bars indicate SD. (D) Correlation of ADA plasma activity with VEGF plasma levels displayed for individual patients (▪, hypoxia) and control patients (•, normoxia). Note the strong correlation between ADA activity and circulating VEGF levels (r2 = .89, P < .01). The diagonal represents the linear regression line.
Plasma ADA enzyme activity is increased in patients with hypoxia. (A) To develop a technique for measuring ADA activity in plasma, different concentrations of adenosine were added to the plasma and assessed for dCF-inhibited inosine generation. Optimal resolution was found with using 20 μM adenosine (black line indicates plasma alone; gray line, plasma after addition of 20 μM adenosine). (B-C) VEGF and ADA are increased in the plasma of patients with hypoxia. Plasma samples were obtained from 10 pediatric patients with chronic hypoxia (mean oxygen saturation of 82%) undergoing cardiac surgery (bidirectional Glenn procedure) and 10 age- and sex-matched controls (mean oxygen generation of 96%) undergoing noncardiac surgery. Plasma VEGF was measured with standard ELISA technique. ADA activity was measured via HPLC as percent adenosine conversion to inosine. *Significant differences with hypoxia (P < .01). #Differences with dCF treatment (100 nM; P < .001). (B-C) Error bars indicate SD. (D) Correlation of ADA plasma activity with VEGF plasma levels displayed for individual patients (▪, hypoxia) and control patients (•, normoxia). Note the strong correlation between ADA activity and circulating VEGF levels (r2 = .89, P < .01). The diagonal represents the linear regression line.
We next extended this analysis to human patients with chronic hypoxia. For this purpose, we obtained plasma samples from 10 pediatric patients undergoing open cardiac surgery (bidirectional Glenn procedure) who had previous oxygen saturations of less than 90% (mean oxygen saturation of 81.9% ± 6%, mean age 51 ± 29 months, 4 male, 6 female; Table 1). Plasma samples were obtained prior to their operation. Ten age- and sex-matched patients undergoing noncardiac surgery (mean oxygen saturation of 96.2% ± 0.8%, mean age 66 ± 30 months, 6 male, 4 female) served as controls. To confirm that the patients undergoing cardiac surgery were indeed hypoxic, we measured VEGF plasma levels. We found a 3.0 ± 0.26-fold increase in plasma VEGF levels compared with the control patients (Figure 6B; P < .01). We also found a 4.1 ± 0.39-fold increase in plasma ADA activity in the cardiac surgery patients (Figure 6C; P < .01). Measured ADA activity was inhibited more than 95% by dCF (Figure 6C; P < .001). Further analysis of these findings in plasma (Figure 6C) showed a strong correlation between ADA activity and circulating VEGF levels (r2 = .89; P < .01). These findings confirm our original hypothesis, and implicate physiologic regulation of plasma ADA enzyme activity by hypoxia in human patients.
Discussion
In these studies, we sought to identify a pathway for the catabolism of extracellular adenosine generated during hypoxia. Our results indicate that hypoxia induces endothelial ADA and CD26 at both the mRNA and protein levels, and that such induced ADA is enzymatically active and tethered on the outside of the membrane via CD26 to form a complex capable of degrading extracellular adenosine. Similarly, plasma ADA activity was increased in vivo in a murine model of acute hypoxia, and in chronically hypoxic pediatric patients, thereby providing an important mechanism to balance extracellular adenosine levels in conditions of high adenine nucleotide metabolism (Figure 7).
The present data indicate, for the first time, a catabolic pathway directed toward limiting vascular adenosine levels in hypoxia. Several previous studies have shown that acute hypoxia is associated with increased vascular adenosine levels.12,13,37 Such increases in vascular adenosine generation and signaling are critical to maintain vascular endothelial function during conditions of acute hypoxia.1,3,5-7 By contrast, chronic elevation of adenosine levels may be associated with excessive adenosine receptor signaling and/or adenosine receptor desensitization, resulting in pulmonary toxicity21 and fibrosis.20 It is now better understood how hypoxia elevates extracellular adenosine. For example, acute hypoxia directly increases levels of the ectonucleotidases CD39 and CD73,3,5,6,24 surface enzymes which coordinate adenosine generation from precursor nucleotides. Both, cd39- and cd73-null mice show increased vascular permeability, tissue edema, and PMN tissue accumulation during acute hypoxia exposure, and such observations implicate protective influences of adenosine on multiple physiologic pathways associated with hypoxia.3,5,6 Moreover, hypoxia also promotes adenosine signaling by selective adenosine receptor induction. In addition, recent studies have also revealed a third mechanism for increasing vascular adenosine signaling, namely hypoxia-mediated repression of the equilibrative nucleoside transporters (ENT1 and ENT2).14 Thus, strong molecular insights now exist to explain the elevation of extracellular adenosine in hypoxia.
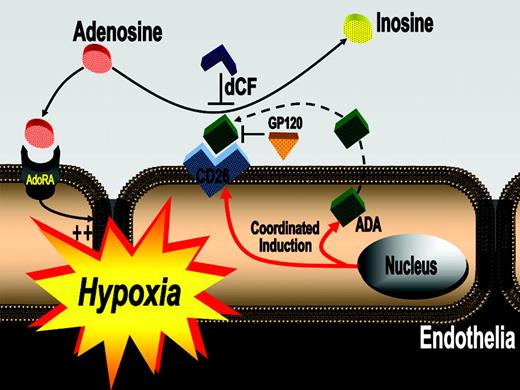
Proposed model of coordinated induction of ADA and CD26. In areas of ongoing inflammation and diminished oxygen supply, hypoxia coordinates the induction of endothelial ADA and CD26. Following induction of ADA mRNA, the enzyme is synthesized and released from the endothelial cell and binds to its cell-surface receptor CD26. Such increases in endothelial cell-surface ADA modulate vascular adenosine signaling during hypoxia. In general, extracellular adenosine can activate endothelial adenosine receptors. Due to increased ADA surface activity with hypoxia, adenosine is metabolized to inosine, thus terminating vascular adenosine signaling and increasing extracellular inosine concentration. Inhibition of ADA with dCF or inhibition of ADA binding to its receptor CD26 (GP120) can contribute to increasing vascular adenosine effects during acute hypoxia.
Proposed model of coordinated induction of ADA and CD26. In areas of ongoing inflammation and diminished oxygen supply, hypoxia coordinates the induction of endothelial ADA and CD26. Following induction of ADA mRNA, the enzyme is synthesized and released from the endothelial cell and binds to its cell-surface receptor CD26. Such increases in endothelial cell-surface ADA modulate vascular adenosine signaling during hypoxia. In general, extracellular adenosine can activate endothelial adenosine receptors. Due to increased ADA surface activity with hypoxia, adenosine is metabolized to inosine, thus terminating vascular adenosine signaling and increasing extracellular inosine concentration. Inhibition of ADA with dCF or inhibition of ADA binding to its receptor CD26 (GP120) can contribute to increasing vascular adenosine effects during acute hypoxia.
However, it is not surprising that hypoxia coordinates the induction of a metabolic pathway that terminates adenosine signaling. Adenosine receptors, like other G-protein-coupled receptors, are tightly regulated by ligand availability.38 With chronic exposure to ligand, regulatory pathways exist to attenuate signaling through these receptors, including both receptor desensitization and receptor internalization mediated by the recruitment of β-arrestins.38-40 Additional studies have shown that following chronic receptor stimulation, β-arrestins also recruit phosphodiesterases to Gαs-coupled receptors (eg, adenosine A2a and A2b receptors), thereby coordinately blocking G-protein signaling and promoting cyclic AMP degradation.41 In a similar fashion, our studies suggest that the induction of adenosine generating capacity (by CD39 and CD73) and adenosine catabolizing capacity (by ADA) may be coordinated. For example, 7- to 10-fold increases in CD39 and CD73 mRNA have been reported in HMEC-1 cells exposed to hypoxia for 8 hours, and maximal increases are 12- to 20-fold.6 In contrast, the induction of endothelial ADA is slower and of lower magnitude (less than 3-fold at 8 hours; Figure 1B). Furthermore, endothelial CD39 is increased to almost 15-fold after only 2 hours of ex vivo hypoxia exposure of saphenous vein tissue.6 In contrast, using the same vascular model, maximum ADA induction occurs after 8 hours with only a nearly 6-fold elevation of its baseline transcript (Figure 1C). These observations suggest that ADA induction by hypoxia and localization to the endothelial cell surface may be delayed compared with hypoxia-associated increases in nucleotide phosphohydrolysis and signaling. Thus, this time course of events suggests that the induction of ADA, as part of the physiologic response to hypoxia, represents a catabolic pathway to prevent long-term increases of extracellular adenosine.
The present study demonstrates that hypoxia coordinates the induction of ADA localized to the cell surface via CD26, thereby increasing the endothelial capacity to metabolize adenosine to inosine. Currently, there is no evidence for distinct ADA mRNA transcripts encoding cytoplasmic versus cell-surface forms of ADA, and the mechanism by which ADA is exported from cells and becomes bound to CD26 is not known. It may be that ADA simply “leaks” out of damaged cells in physiologic situations such as hypoxia or inflammation. However, at the same time, hypoxia promotes extracellular inosine production and may promote inosine signaling. To date, no specific inosine receptors have yet been characterized. However, Jin et al42 observed that that like adenosine, inosine can also elicit vasoconstriction of hamster cheek pouch arterioles via mast cell degranulation as a consequence of A3 adenosine receptor binding. In the present study, specific effects of inosine generation related to increased ADA activity were not investigated.
As part of our analyses, we examined circulating levels of ADA in a murine model of hypoxia and in chronically hypoxic human subjects (patients undergoing bidirectional Glenn procedure to repair congenital heart defects). In both cases, there was a strong correlation (P < .01) between the serum hypoxic marker VEGF and increased circulating levels of enzymatically active ADA. Murine ADA does not bind to murine CD26.43 Therefore, at the present time, we do not know whether or not there is also increased surface-localized ADA in murine models of hypoxia. There are reports in the literature that ADA can also be tethered to the cell surface via A144 and A2b45 adenosine receptors. However, these findings have yet to be replicated by other groups. Certainly, in our human studies, ADA is anchored to the cell membrane via CD26 since the association of ADA with the cell surface can be disrupted by treatment with HIV gp120, a protein that is known to compete with ADA for binding to CD26.30-32
Our studies with chronically hypoxic children revealed that an approximately 15% decrease in mean oxygen saturation resulted in a more than 4-fold increase in circulating ADA levels. It is important to note that circulating adenosine has strong cardiac influences. In particular, of its many central nervous actions, adenosine can induce hypotension and cardiac suppression,46 and thus, it is tempting to speculate that an important adaptive pathway for these patients is rapid adenosine clearance. Such clearance could be achieved through the ADA induction pathway we describe here. Further studies are necessary to elucidate whether these patients benefit in other ways from increased circulating ADA levels.
In summary, these results identify ADA induction by hypoxia as an innate adaptation of the vascular endothelium to compensate for elevated extracellular adenosine levels. Through coordinated induction of the ADA-complexing protein CD26, ADA localizes to the endothelial surface and limits the accumulation of extracellular adenosine during hypoxia. Extensions of these findings will determine whether ADA may serve as a therapeutic target for disorders involving vascular leak syndrome or excessive inflammatory responses during acute hypoxia.
Prepublished online as Blood First Edition Paper, May 2, 2006; DOI 10.1182/blood-2006-02-001016.
Supported by Fortune grants 1416-0-0 and 1319-0-0, Interdisziplinäres Zentrum für Klinische Forschung (IZKF) Verbundprojekt and Deutsche Forschungsgesellschaft (DFG) grant EL274/2-2 (H.K.E.), DFG grant KA 2330/1-2 (J.K.), and by National Institutes of Health (NIH) grants HL60569 and DK50189 (S.P.C.) and AI 18220 and HD 36044 (L.F.T).
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to acknowledge Alice Mager, Michaela Hochgutbrot, Stephanie Zug, and Edgar Hoffmann for technical assistance, and Shelley K. Eltzschig for artwork during manuscript preparation.

![Figure 2. ADA induced by hypoxia is enzymatically active. (A) To measure ADA enzyme activity on the endothelial surface, HMEC-1s were exposed over indicated time periods to hypoxia (pO2 20 mm Hg), 50 μM adenosine was added to the supernatant (HBSS) of intact HMEC-1s, and inosine generation was measured via HPLC. The HPLC tracing obtained from the supernatant is displayed and retention times for inosine (3.2 min) and for adenosine (3.7 min) are indicated (black line indicates HBSS alone; gray line, HBSS after addition of adenosine [50 μM]). (B) Induction of ADA activity in total-cell lysates of endothelia by hypoxia. To obtain an estimate of total ADA activity increase with hypoxia, the ADA activity in lysates of HMEC-1s was measured. (C) Induced, enzymatically active ADA is localized to the cell surface. ADA enzyme activity was measured in intact HMEC-1s by adding 50 μM adenosine to the supernatant (HBSS) and measuring inosine generation. All experiments were performed in the presence of 10 μM dipyridamole to prevent endothelial adenosine uptake. To confirm that the observed increase in inosine generation with hypoxia exposure reflects ADA activity, controls with 100 nM dCF were performed in normoxic (dCF [Norm]) or posthypoxic (dCF [Hyp 48 h]) endothelia. Note the robust hypoxia induction of functional ADA to the cell surface. (D) ADA release into the supernatant is increased with hypoxia. To measure ADA release into the supernatant, HMEC-1s were exposed to normoxia or hypoxia (HMEC-1, pO2 20 mm Hg, 48 hours). During the last 4 hours, the media was replaced with HBSS and ADA activity in the cell supernatant was measured. In controls, dCF (dCF [Hyp 48 h], 100 nM) inhibited soluble ADA activity in posthypoxic supernatants. In contrast, the HIV-1 envelope glycoprotein gp120 (gp120 [Hyp 48 h], 100 nM) did not affect ADA activity in the supernatant. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2006-02-001016/2/m_zh80170600340002.jpeg?Expires=1768233638&Signature=dvnHY0Oa8UmjtK7MeffKVvYz0hPG1Dwwx0vIxDPZIvngBO3e-Fe8ETR9AbPiEsuGjhhaucGpljGcsDe5AEWsdNVb25kh0L1qPXZ8GxzdbnZjOOlPh6BvXUBRIpRdzHCZFvVN-tpo5ZQWJ3p2Wg3lI~GIWVgzWxdLZ36XuX5wV1ogU8RZXtZ~bMsD5ZLprXivmQlsvv~gagIToD7AgOSeTifyyXdoqdE9vZjyvWNn1jYHNL1J8vHBEc4wg3bdUPImSrDQnTsX7DC8Mf6-iVG~KzPWS3b~IRZ7M9OvgtlLRli1On6qM7rh6nzWW4Tsv4Lkh2qMuqp3FC6MOWHHlhzcAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. ADA induced by hypoxia is enzymatically active. (A) To measure ADA enzyme activity on the endothelial surface, HMEC-1s were exposed over indicated time periods to hypoxia (pO2 20 mm Hg), 50 μM adenosine was added to the supernatant (HBSS) of intact HMEC-1s, and inosine generation was measured via HPLC. The HPLC tracing obtained from the supernatant is displayed and retention times for inosine (3.2 min) and for adenosine (3.7 min) are indicated (black line indicates HBSS alone; gray line, HBSS after addition of adenosine [50 μM]). (B) Induction of ADA activity in total-cell lysates of endothelia by hypoxia. To obtain an estimate of total ADA activity increase with hypoxia, the ADA activity in lysates of HMEC-1s was measured. (C) Induced, enzymatically active ADA is localized to the cell surface. ADA enzyme activity was measured in intact HMEC-1s by adding 50 μM adenosine to the supernatant (HBSS) and measuring inosine generation. All experiments were performed in the presence of 10 μM dipyridamole to prevent endothelial adenosine uptake. To confirm that the observed increase in inosine generation with hypoxia exposure reflects ADA activity, controls with 100 nM dCF were performed in normoxic (dCF [Norm]) or posthypoxic (dCF [Hyp 48 h]) endothelia. Note the robust hypoxia induction of functional ADA to the cell surface. (D) ADA release into the supernatant is increased with hypoxia. To measure ADA release into the supernatant, HMEC-1s were exposed to normoxia or hypoxia (HMEC-1, pO2 20 mm Hg, 48 hours). During the last 4 hours, the media was replaced with HBSS and ADA activity in the cell supernatant was measured. In controls, dCF (dCF [Hyp 48 h], 100 nM) inhibited soluble ADA activity in posthypoxic supernatants. In contrast, the HIV-1 envelope glycoprotein gp120 (gp120 [Hyp 48 h], 100 nM) did not affect ADA activity in the supernatant. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2006-02-001016/2/m_zh80170600340002.jpeg?Expires=1768233639&Signature=EsR1x9f~5qz-NfrEyxiUTsluPTbjSE8YZO-~cvkmTFnv8~89ZGok3SvvXaCuh-eOjGrC2UHq~j63h~zZC8pc1IM8f923cIfd0NI-BIQDKOZ4nu4jlXUX6YYNFn7ZcpbnIyxSxV2pgDtCBLaH4McJ1TZl-VfPpeFSjSaJkbg-g~Phe5wucJvaFQ0Ic7flEWxvBXuXz5yaLckqw8nf9KGqh2h2QbkBLZkizzCIaEI2WUniY-Tic98hHPaAdEZDvetkKwJ57vw2a0-5B5vaXG8KMjp4~hd7MusE1VJsBP7YSOAVJPnVrIc9cYmeINtOIcgxFCmDvfZWLNr8PdgM82Y1ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ) after dCF or PBS treatment. Data are expressed as mean ± SD mg H2O/mg dry tissue, and are pooled from 6 animals per condition. *Significantly different between hypoxia and normoxia (P < .025). #Significantly different between dCF treatment and vehicle control (P < .025). (B) To assess vascular barrier function, animals were administered intravenous Evan blue dye solution (0.2 mL of 0.5% in PBS) prior to normoxia/hypoxia exposure. Animals were killed, and the lung, colon, and liver were harvested. Organ Evan blue concentrations were quantified following formamide extraction (55°C for 2 hours) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Data are expressed as mean ± SD Evan blue optical density (OD)/50mg wet tissue, and are pooled from 6 animals per condition. Note that Evan blue retention increases with hypoxia and decreases with dCF treatment. *Significant differences between normoxia/hypoxia exposure (P < .01). #Significant differences between dCF/PBS treatment groups (P < .025). (C) Organ assessment of PMN accumulation by myeloperoxidase (MPO) measurements in the indicated organs after 4 hours of normoxia/hypoxia exposure (*P < .01 compared with hypoxia; #P < .025 compared with vehicle control). Error bars indicate SD.
) after dCF or PBS treatment. Data are expressed as mean ± SD mg H2O/mg dry tissue, and are pooled from 6 animals per condition. *Significantly different between hypoxia and normoxia (P < .025). #Significantly different between dCF treatment and vehicle control (P < .025). (B) To assess vascular barrier function, animals were administered intravenous Evan blue dye solution (0.2 mL of 0.5% in PBS) prior to normoxia/hypoxia exposure. Animals were killed, and the lung, colon, and liver were harvested. Organ Evan blue concentrations were quantified following formamide extraction (55°C for 2 hours) by measuring absorbances at 610 nm with subtraction of reference absorbance at 450 nm. Data are expressed as mean ± SD Evan blue optical density (OD)/50mg wet tissue, and are pooled from 6 animals per condition. Note that Evan blue retention increases with hypoxia and decreases with dCF treatment. *Significant differences between normoxia/hypoxia exposure (P < .01). #Significant differences between dCF/PBS treatment groups (P < .025). (C) Organ assessment of PMN accumulation by myeloperoxidase (MPO) measurements in the indicated organs after 4 hours of normoxia/hypoxia exposure (*P < .01 compared with hypoxia; #P < .025 compared with vehicle control). Error bars indicate SD.