IL-23, an IL-12-related cytokine, induces an IL-17-secreting T-helper phenotype that is involved in autoimmune diseases and host defense against certain pathogens. Although the transcription factors required for development of IL-23-stimulated cells are unknown, we show that T-bet is a critical negative regulator of the IL-23-primed T-cell phenotype, which we term Th1β. Th1 or Th1β Tbx21-/- cultures secrete higher than WT levels of IL-17 in response to T-cell receptor (TCR) or IL-23 + IL-18 stimulation. Ectopic T-bet expression in Th1β cells promotes IFN-γ secretion but decreases IL-17 production. Although antigen-receptor stimulation of Th1β cells stimulates IL-17 production, it also induces the IFN-γ-independent expression of T-bet and progression to a Th1 cytokine secretion pattern. T-bet is required for the progression to the Th1 phenotype, because Tbx21-/- Th1β cultures maintain the IL-17-secreting phenotype after 2 weeks of culture. Addition of IFN-γ to Tbx21-/- Th1β cultures cannot recover the progression to the Th1 phenotype, suggesting T-bet, rather than IFN-γ, mediates Th1β to Th1 progression. The transient nature of the Th1β phenotype suggests that these cells are a component of type I immunity and that T-bet expression is a critical determinant of Th1 versus Th1β cell fate.
Introduction
IL-23, an IL-12-related cytokine, shares structural and functional similarity with IL-12. Both cytokines are heterodimers that share a common IL-12p40 subunit linked to the IL-12p35 chain or the IL-23p19 chain.1 IL-23 functions through a receptor complex composed of the IL-12Rβ1 subunit and a unique IL-23R chain.2 Both cytokines induce IFN-γ expression in CD4+ T cells. and dendritic cells,3,4 although only IL-23 facilitates a T-helper state marked by production of the proinflammatory cytokine, IL-17.5-8 Th cells primed for IL-17 production appear to have important roles in autoimmune diseases.6,9 These studies suggest that IL-12 and IL-23 have distinct roles in promoting cell-mediated immune responses and autoimmune diseases in vivo.
Recent analysis of this novel Th subset demonstrated that development in vivo depends on ICOS and CD28 but not on Stat4 or Stat6.8 In vitro differentiation of this subset is inhibited by IL-4 and IFN-γ and occurs in the absence of Stat1, Stat4, or Stat6.7,8 Both reports suggested that T-bet, a transcription factor that is critical for the development of Th1 cells and IFN-γ secretion, decreases IL-17 production in IL-23-primed Th cells.7,8,10,11 However, it is unclear whether T-bet inhibits the IL-23-induced Th phenotype directly or indirectly by the induction of IFN-γ. Moreover, the stability of this phenotype has not been well documented.
In this report, we have explored the negative regulatory role of T-bet in the cell fate decisions between IL-12- and IL-23-primed cells. We demonstrate that T-bet expression levels are a critical determinant of cell phenotype. Tbx21-/- (T-bet-deficient) cultures secrete higher levels of IL-17 than WT cultures in response to T-cell receptor (TCR) and cytokine stimulation. Moreover, increased T-bet expression, either by retroviral transduction or by secondary TCR stimulation of WT but not Tbx21-/- IL-23-primed cultures, results in a progression to a Th1 phenotype. The transient nature of the IL-23-primed phenotype suggests that these cells are a component of type I immunity that we refer to as Th1β cells. These data also define T-bet expression as a central factor in Th1 versus Th1β cell fate.
Materials and methods
Analysis of Th1 and Th1β T cells
The generation of Tbx21-/- (T-bet-deficient) mice were previously described.12 Tbx21-/- mice were either supplied by Dr L. Glimcher (Harvard University School of Public Health, Boston, MA) or obtained from Jackson Laboratories (Bar Harbor, ME). CD4+ T cells were isolated from lymph nodes and splenocytes of 8- to 10-week-old Tbx21-/- Balb/C or C57BL/6 mice and strain-matched wild-type controls (magnetic cell sorting [MACS] isolation system; Miltenyi Biotec, Auburn, CA). Cells (1 × 106 cells/mL) were activated with plate-bound α-CD3 (2C11 clone; 2-4 μg/mL) and α-CD28 (BD Pharmingen, San Diego, CA; 0.5 μg/mL) and cultured in RPMI 1640 (Invitrogen, Rockville, MD) supplemented as described.13 Th1 or Th1β cells were differentiated with IL-12 (PeproTech, Rocky Hill, NJ; 2 ng/mL) and α-IL-4 (11B11 clone; 10 μg/mL) or IL-23 (R&D Systems, Minneapolis, MN; 4 ng/mL) and α-IL-4, respectively, for 3 days and expanded in the presence of IL-12 or IL-23 for 2 more days. An aliquot of cells was then washed and stimulated at 1 × 106 cells/mL for 18 to 24 hours with medium alone, α-CD3 (2-4 μg/mL), IL-12 (2 ng/mL), IL-18 (25 ng/mL; PeproTech), IL-23 (4 ng/mL), IL-12 + IL-18, or IL-23 + IL-18. Remaining cells were diluted to 3 × 105 cells/mL and cultured with or without α-CD3 (0.5 μg/mL) and IL-12 or IL-23 for 3 days and expanded for 2 additional days in the presence of IL-12 or IL-23. Some cultures were supplemented with α-IFN-γ (R4/6A2 from BD Pharmingen or isolated by hybridoma and XMG-1 from BD Pharmingen) or IFN-γ (PeproTech) as noted in figures. Live cells were obtained from Histopaque gradient (Sigma Aldrich, St Louis, MO) and plated at 1 × 106 cells/mL with or without α-CD3 (2 μg/mL) for 24 hours. Cell-free supernatants from both time points were analyzed simultaneously for IL-17 or IFN-γ production using enzyme-linked immunosorbent assay (ELISA; antibodies from BD Pharmingen, cytokine standards from R&D Systems). In preliminary experiments, a mouse IL-23-Ig fusion4 was compared in assays with human IL-23 (R&D Systems) for bioactivity and to determine the optimal concentration of IL-23. All animal studies were approved by the Indiana University Institutional Animal Care and Use Committee.
Immunoblotting
Whole-cell protein lysates from freshly isolated CD4+ T cells or 5-day differentiated Th1, Th1β, or unskewed T cells (treated with 10 μg/mL α-IL-4 only) were stimulated as described in “Results.” Lysate (50-100 μg) was electrophoresed in a 4% to 12% gradient polyacrylamide gel (Invitrogen) and immunoblotted with the 4B10 anti-T-bet antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were counterblotted for β-actin expression (Calbiochem, San Diego, CA).
Cell surface marker staining
Five-day differentiated Th1 or Th1β cells were analyzed for IL-12Rβ2 (Santa Cruz Biotechnology), IL-23R, or IL-18Rα (R&D Systems) as described.13 A PE antigoat secondary antibody for IL-12Rβ2 staining was obtained from Calbiochem (West Grove, PA), and a streptavidin PE secondary antibody for IL-23R and IL-18Rα was obtained from BD Pharmingen.
Intracellular cytokine staining
CD4+ T cells from C57BL/6 splenocytes were cultured under Th1β conditions for 5 days as described in “Analysis of Th1 and Th1β T Cells.” Cells were left unstimulated or stimulated with plate-bound α-CD3 (10 μg/mL) and soluble α-CD28 (1 μg/mL) for 5 hours with Golgiplug (BD Pharmingen). Cells were fixed with 4% paraformaldehyde for 20 minutes and permeablized in PBS containing 0.1% Igepal by volume. Cells were stained with antibodies labeled with PE anti-IL-17 and FITC anti-IFN-γ (BD Pharmingen).
Real-time PCR
RNA from wild-type and Tbx21-/- Th1 and Th1β cells was isolated using TRIzol Reagent (Invitrogen). cDNA was generated from RNA using the First-Strand Cloned AMV kit (Invitrogen). Message levels were analyzed by TaqMan polymerase chain reaction (PCR) (all reagents were obtained from Applied Biosystems, Foster City, CA). Cycle number of duplicate samples was normalized to expression of β2-microglobulin. Results are relative to the wild-type Th1-unstimulated condition, which is set equal to one.
Retroviral transfections
Results
TCR stimulation of Th1β cells promotes a switch from an IL-17-secreting to an IFN-γ-secreting phenotype
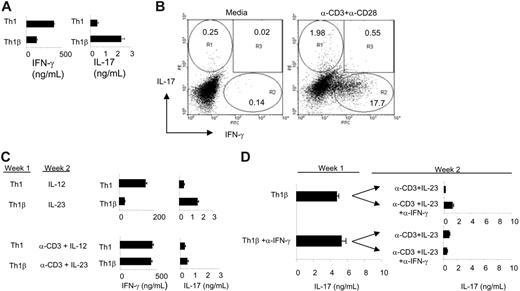
Previous studies demonstrated that incubation of CD4+ T cells with IL-23 results in a differentiated T-cell state primed for IL-17 and IFN-γ production.3,5 Although IL-12 promotes a stable Th1 phenotype marked by secretion of IFN-γ, it is unclear whether IL-23 similarly induces a heritable IL-17-secreting T-cell state. To determine whether IL-23-primed T cells stably secrete IL-17, primary murine CD4+ T cells were activated with α-CD3 and α-CD28 and cultured under Th1 (IL-12 + α-IL-4) or Th1β (IL-23 + α-IL-4) conditions (Figure 1). At day 5, differentiated cells were washed and restimulated with α-CD3 or left unstimulated for 24 hours, and cell-free supernatants were collected to analyze cytokine production (Figure 1A). In agreement with previously published reports, Th1 cells are primed for IFN-γ production, whereas Th1β cells secrete higher levels of IL-17 compared with Th1 cultures. As shown in Figure 1B and in several other systems,6-8 intracellular cytokine staining reveals that IL-23-cultured cells are predominantly single cytokine (IL-17 or IFN-γ) producers.
In parallel, 5-day differentiated cells were replated with IL-12 or IL-23 in the presence or absence of plate-bound α-CD3 for 5 additional days. After the culture period, live cells were washed and left unstimulated or restimulated with plate-bound α-CD3 for 24 hours, and cell-free supernatants were analyzed for IL-17 and IFN-γ production using ELISA (Figure 1C). Cytokine levels from the unstimulated cell populations were undetectable by these assays (data not shown). Although IL-23 induces a T-cell state primed for IL-17 secretion during the first week of culture (Figure 1A), antigen-receptor stimulation during the second week of culture promotes a progression of IL-23-cultured cells to a population that secretes IFN-γ in quantities comparable to Th1 cells (Figure 1C). The shift in cytokine secretion pattern requires antigen receptor stimulation because Th1β cells that were incubated with cytokine alone for the second week maintained the IL-17-secreting phenotype (Figure 1C). These data suggest that the IL-17-secreting T-cell state is not a stable or heritable phenotype. As the IL-17-secreting phenotype is transient and defaults to a Th1 phenotype following subsequent antigen receptor stimulation, we refer to the IL-23-differentiated phenotype as Th1β.
Because Th1β cells secrete IFN-γ (Figure 1A), and IFN-γ decreases Th1β development,7,8 we hypothesized that IFN-γ neutralization would recover IL-17 production. To test this, activated CD4+ T cells were differentiated for 5 days (week 1) under Th1β conditions with or without α-IFN-γ and restimulated as in Figure 1A. IL-17 levels were measured using ELISA (Figure 1D). In parallel, 5-day differentiated cells were cultured with α-CD3 and IL-23 for 5 additional days in the presence or absence of IFN-γ-neutralizing antibodies. IFN-γ neutralization was confirmed using ELISA. After the first week, Th1β cells differentiated in the presence or absence of α-IFN-γ had comparable levels of IL-17 following TCR stimulation. Although IFN-γ neutralization during the first, second, or both weeks of differentiation did have modest effects on increasing IL-17 levels, neutralization is not sufficient to restore IL-17 production to levels observed after only 1 week of culture (Figure 1D). We conclude that IFN-γ neutralization is not sufficient to maintain IL-17 secretion in long-term Th1β cultures.
IL-23-cultured CD4+ T cells transiently secrete IL-17 and progress to a Th1 cytokine-secreting profile. (A) CD4+ T cells from C57BL/6 mice were activated with α-CD3 and α-CD28 and cultured with α-IL-4. IL-12 or IL-23 was added to the culture to generate Th1 or Th1β cells, respectively. Cells were cultured for 3 days and expanded in the presence of IL-12 or IL-23 for 2 additional days. After 5 days, cells were left unstimulated or were restimulated with α-CD3 for 24 hours, and cell-free supernatants were analyzed for IL-17 and IFN-γ secretion using ELISA. (B) Th1β cells were cultured for 5 days as in panel A. Cells were replated with media alone or restimulated with α-CD3 and α-CD28 for 5 hours in the presence of Brefeldin A. Cells were fixed, permeabilized, and stained with PE-α-IL-17 and FITC-α-IFN-γ antibodies and analyzed for intracellular cytokine staining by flow cytometry. Numbers indicate percentage of cells within each region. (C) Th1 or Th1β cells differentiated for 5 days (week 1) in panel A were cultured for 5 additional days (week 2) with or without plate-bound α-CD3. Th1 and Th1β cultures were maintained in media containing IL-12 or IL-23, respectively. At day 10, live cells were cultured in the presence or absence of plate-bound α-CD3 for 24 hours, and supernatants were analyzed for IFN-γ and IL-17 production using ELISA. Unstimulated cultures at day 10 did not produce detectable levels of cytokine. (D) Five-day Th1β cultures differentiated in the presence or absence of α-IFN-γ (10 μg/mL) were restimulated as in panel A, and cell-free supernatants were measured for IL-17 quantities using ELISA (week 1). In parallel, 5-day differentiated cells were cultured for 5 more days (week 2) in the presence of plate-bound α-CD3 and maintained in media containing IL-23 with or without α-IFN-γ as indicated in the figure. Two-week cultures were restimulated as in panel C, and IL-17 levels from cell-free supernatants were measured using ELISA. ELISA data are represented as mean ± SE of replicate samples.
IL-23-cultured CD4+ T cells transiently secrete IL-17 and progress to a Th1 cytokine-secreting profile. (A) CD4+ T cells from C57BL/6 mice were activated with α-CD3 and α-CD28 and cultured with α-IL-4. IL-12 or IL-23 was added to the culture to generate Th1 or Th1β cells, respectively. Cells were cultured for 3 days and expanded in the presence of IL-12 or IL-23 for 2 additional days. After 5 days, cells were left unstimulated or were restimulated with α-CD3 for 24 hours, and cell-free supernatants were analyzed for IL-17 and IFN-γ secretion using ELISA. (B) Th1β cells were cultured for 5 days as in panel A. Cells were replated with media alone or restimulated with α-CD3 and α-CD28 for 5 hours in the presence of Brefeldin A. Cells were fixed, permeabilized, and stained with PE-α-IL-17 and FITC-α-IFN-γ antibodies and analyzed for intracellular cytokine staining by flow cytometry. Numbers indicate percentage of cells within each region. (C) Th1 or Th1β cells differentiated for 5 days (week 1) in panel A were cultured for 5 additional days (week 2) with or without plate-bound α-CD3. Th1 and Th1β cultures were maintained in media containing IL-12 or IL-23, respectively. At day 10, live cells were cultured in the presence or absence of plate-bound α-CD3 for 24 hours, and supernatants were analyzed for IFN-γ and IL-17 production using ELISA. Unstimulated cultures at day 10 did not produce detectable levels of cytokine. (D) Five-day Th1β cultures differentiated in the presence or absence of α-IFN-γ (10 μg/mL) were restimulated as in panel A, and cell-free supernatants were measured for IL-17 quantities using ELISA (week 1). In parallel, 5-day differentiated cells were cultured for 5 more days (week 2) in the presence of plate-bound α-CD3 and maintained in media containing IL-23 with or without α-IFN-γ as indicated in the figure. Two-week cultures were restimulated as in panel C, and IL-17 levels from cell-free supernatants were measured using ELISA. ELISA data are represented as mean ± SE of replicate samples.
T-bet negatively regulates Th1β development
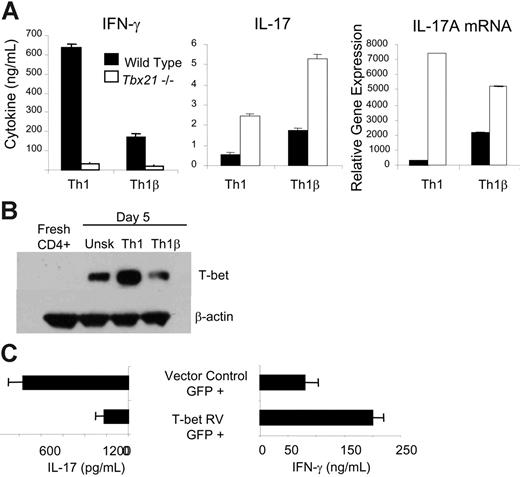
To understand the distinct cytokine profiles produced by Th1 and Th1β cultures during the first week of differentiation, as well as the progression of Th1β cells to a Th1 phenotype, we speculated that a promoter of the Th1 phenotype could be a negative regulator of Th1β differentiation. Recent reports have suggested that T-bet negatively regulates IL-23-stimulated differentiation as it does Th2 development.7,8,10 To determine whether T-bet negatively regulates IL-17 production from Th1 and Th1β cultures, wild-type and Tbx21-/- (T-bet deficient) CD4+ T cells were differentiated under Th1 or Th1β conditions for 5 days, washed, and left unstimulated or stimulated with α-CD3. IFN-γ and IL-17 concentrations were measured from cell-free supernatants using ELISA, and IL-17A mRNA expression was assessed by real-time PCR (Figure 2A). Although T-bet is required for antigen receptor-stimulated IFN-γ secretion for both Th1 and Th1β cells, quantities of IL-17 are higher in T-bet-deficient T cells, even under Th1 culture conditions, suggesting that, although T-bet promotes IFN-γ production, it negatively regulates IL-17 secretion and mRNA expression (Figure 2A).
The ability of T-bet to decrease Th1β differentiation suggests that Th1β cells must maintain lower levels of T-bet than Th1 cells. We tested this with immunoblot analysis of total cell extracts from fresh CD4+ T cells and activated cells differentiated for 5 days under unskewed (α-IL-4 alone), Th1, or Th1β conditions. Although all culture conditions generate increased expression of T-bet compared with fresh CD4+ T cells, Th1β cells express comparable levels of T-bet to unskewed T cells and considerably lower levels than Th1 cells (Figure 2B). These data led us to predict that ectopic T-bet expression in Th1β cells would induce cells primed for IFN-γ expression at the expense of IL-17 secretion. To test this, Th1β cells were infected on day 2 of development with bicistronic retroviruses expressing T-bet and EGFP or EGFP alone. Cells were cultured for 3 more days, sorted for EGFP-positive cells, and stimulated with α-CD3 for 18 hours. Cell-free supernatants were analyzed for IL-17 and IFN-γ secretion using ELISA (Figure 2C). Ectopic T-bet expression in Th1β cells induced IFN-γ production and diminished levels of IL-17, a cytokine secretion pattern similar to wild-type Th1 cells. Thus, increased T-bet expression can promote progression from a Th1β to a Th1 phenotype.
T-bet negatively regulates cytokine-induced IL-17 secretion
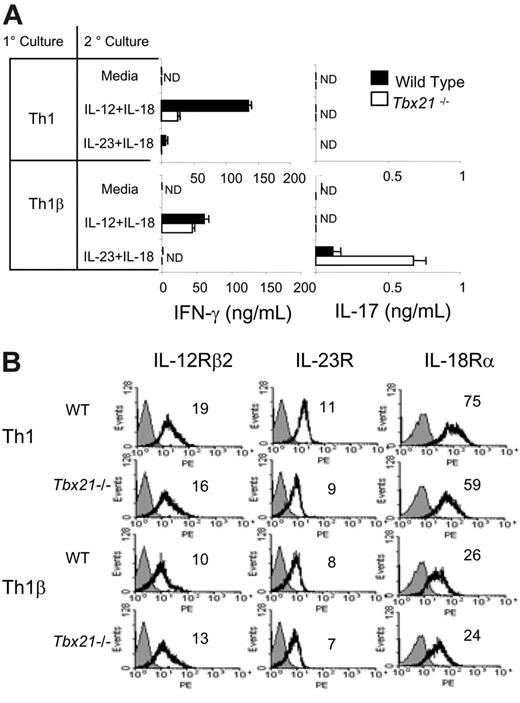
The ability of T-bet to decrease antigen-receptor-induced IL-17 production suggested that it might also affect cytokine-induced IL-17 secretion. To test the ability of IL-23 to synergize with IL-18, as IL-18 does with IL-12 in the induction of IFN-γ,16 we restimulated 5-day cultured Th1 and Th1β cells (primary culture) with media alone, IL-12 + IL-18, or IL-23 + IL-18 (secondary culture) and assayed for the production of IFN-γ and IL-17 using ELISA. Although restimulation with individual cytokines alone had little effect (data not shown), stimulation with IL-12 + IL-18 induces production of IFN-γ from Th1 cells and to a lesser degree from Th1β cells (Figure 3A). IL-23 + IL-18 stimulate IL-17 production only in Th1β cells while promoting minimal IFN-γ production from either Th1 or Th1β cells (Figure 3A). Moreover, IL-23 + IL-18 stimulates increased IL-17 secretion from T-bet-deficient Th1β cells but not from Tbx21-/- Th1 cells. This is in contrast to the ability of α-CD3 stimulation to promote IL-17 production in Tbx21-/- Th1 cultures (Figure 2A), suggesting that Tbx21-/- Th1 cultures are not phenotypically identical to Tbx21-/- Th1β cultures. These results further support the critical role of T-bet in modulating IL-23-mediated T-cell development and suggest that the ability of IL-18 to promote type I immunity might include functions coupled to IL-23, as well as IL-12.
T-bet negatively regulates Th1β development. (A) WT and Tbx21-/- CD4+ T cells were cultured under Th1 (IL-12 + α-IL-4) or Th1β (IL-23 + αx-IL-4) conditions for 5 days and restimulated with α-CD3 for 18 hours. Cell-free supernatants were analyzed for IFN-γ or IL-17 using ELISA. Unstimulated populations did not produce detectable levels of cytokine. RNA was extracted from T cells activated for 18 hours, and duplicate samples were quantified by real-time PCR for IL-17A mRNA. Cycle number was normalized to β2-microglobulin mRNA and is represented as fold induction relative to unstimulated wild-type Th1 cells. ND indicates not detected. (B) WT CD4+ T cells from C57BL/6 mice were cultured with α-IL-4 alone (Unsk.) or under Th1 or Th1β conditions for 5 days as described in Figure 1A. Protein lysates from freshly isolated CD4+ T cells or differentiated cells were assayed for T-bet expression by immunoblot. (C) WT Th1β cells were cultured for 2 days and infected with a bicistronic retrovirus expressing EGFP alone (vector control) or T-bet and EGFP (T-bet RV). Cells were cultured for 3 more days, and EGFP-positive cells were sorted and restimulated with α-CD3 or left unstimulated for 18 hours. Cell-free supernatants were analyzed for IL-17 and IFN-γ production. Unstimulated cell populations did not produce detectable cytokine levels. ELISA data are represented as mean ± SE of replicate samples.
T-bet negatively regulates Th1β development. (A) WT and Tbx21-/- CD4+ T cells were cultured under Th1 (IL-12 + α-IL-4) or Th1β (IL-23 + αx-IL-4) conditions for 5 days and restimulated with α-CD3 for 18 hours. Cell-free supernatants were analyzed for IFN-γ or IL-17 using ELISA. Unstimulated populations did not produce detectable levels of cytokine. RNA was extracted from T cells activated for 18 hours, and duplicate samples were quantified by real-time PCR for IL-17A mRNA. Cycle number was normalized to β2-microglobulin mRNA and is represented as fold induction relative to unstimulated wild-type Th1 cells. ND indicates not detected. (B) WT CD4+ T cells from C57BL/6 mice were cultured with α-IL-4 alone (Unsk.) or under Th1 or Th1β conditions for 5 days as described in Figure 1A. Protein lysates from freshly isolated CD4+ T cells or differentiated cells were assayed for T-bet expression by immunoblot. (C) WT Th1β cells were cultured for 2 days and infected with a bicistronic retrovirus expressing EGFP alone (vector control) or T-bet and EGFP (T-bet RV). Cells were cultured for 3 more days, and EGFP-positive cells were sorted and restimulated with α-CD3 or left unstimulated for 18 hours. Cell-free supernatants were analyzed for IL-17 and IFN-γ production. Unstimulated cell populations did not produce detectable cytokine levels. ELISA data are represented as mean ± SE of replicate samples.
To determine whether any of the differences in cytokine responsiveness in these cultures is due to altered cytokine-receptor expression patterns between Th1 and Th1β cells and whether receptor levels are differentially regulated by T-bet, WT and Tbx21-/- cells were analyzed by flow cytometry (Figure 3B). Th1β cells have slightly lower levels of IL-12Rβ2 expression than Th1 cells, although expression is not dependent on T-bet. Th1 cells have slightly higher levels of IL-23R than Th1β cells, and expression was partially T-bet dependent. Expression of either receptor is not affected by α-CD3 or the cytokine restimulations described in Figure 3A (not shown). Although Th1β cells express lower levels of IL-18Rα compared with Th1 cells, the absence of T-bet does not augment IL-18Rα levels in either Th1 or Th1β cultures, indicating that T-bet is not a determinant of IL-18Rα expression. Subsequent α-CD3 or IL-23 + IL-18 restimulation did not change the expression of IL-18Rα on WT or Tbx21-/- Th1 or Th1β cells (not shown). Thus, the ability of cytokines to induce IL-17 or IFN-γ secretion from Th1 and Th1β cells is unlikely because of differences in cytokine-receptor expression.
T-bet is required for the progression of Th1β cells to a Th1 phenotype
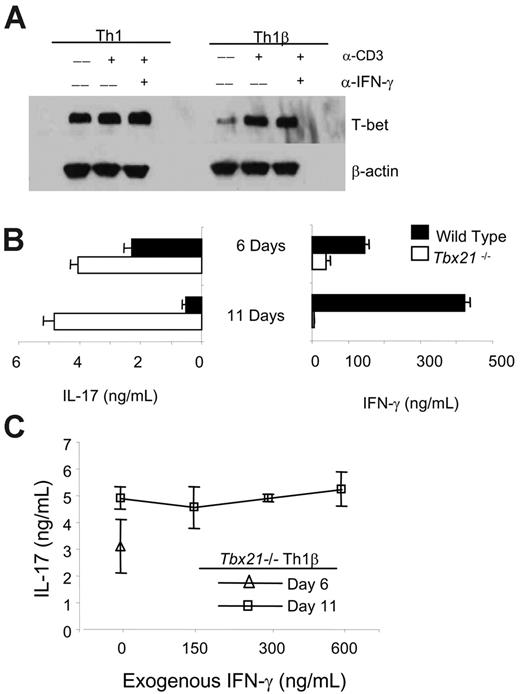
The progression of the Th1β subset to the Th1 phenotype following subsequent antigen-receptor stimulation, suggests that T-bet, which is critical for development of the IFN-γ-secreting phenotype, would mediate this shift in cytokine profile. To test this, we assessed T-bet expression by immunoblot in Th1 and Th1β cells following α-CD3 stimulation. Although α-CD3 did not induce T-bet protein levels in Th1 cells at this time point, T-bet levels in Th1β cells were induced to levels equal to those observed in Th1 cells. This induction was not due to IFN-γ secretion because incubation with α-IFN-γ did not diminish the induction of T-bet by α-CD3 (Figure 4A). This demonstrates that α-CD3 induces T-bet expression in Th1β cells to levels similar to those in Th1 cells and that this induction does not require IFN-γ.
Given the ability of T-bet to shift the phenotype of Th1β cells (Figure 2C) and the observation that α-CD3 restimulation induces T-bet expression (Figure 4A), we hypothesized that TCR stimulation induced T-bet to levels necessary to inhibit IL-17 production and promote IFN-γ secretion in Th1β cultures (Figure 1C), and that this response would therefore be lacking in Tbx21-/- cultures. To test this, wild-type and Tbx21-/- Th1β cells were cultured and analyzed as described for Figure 1A and C. Although wild-type Th1β cells transiently secrete IL-17 and switch to an IFN-γ-secreting profile after secondary stimulation, Tbx21-/- Th1β cells remain primed for IL-17 secretion, suggesting that T-bet is an essential mediator in reverting Th1β cells to an IFN-γ-secreting state and in determining the stability of the Th1β phenotype (Figure 4B).
T-bet negatively regulates cytokine-induced IL-17 production but does not augment cytokine receptor expression on Th1 or Th1β cells. (A) WT or Tbx21-/- Th1 or Th1β cells were cultured for 5 days as in Figure 1A (1° culture). Cells were restimulated with media alone or the indicated cytokines (2° culture) for 18 hours and analyzed for IFN-γ or IL-17 production using ELISA. ND indicates not detected. (B) WT or Tbx21-/- Th1 or Th1β cells were cultured as in Figure 1A. IL-12Rβ2, IL-23R, or IL-18Rα expression was assessed using flow cytometry. Numbers within each histogram represent mean fluorescent intensities averaged from 2 independent experiments. Black line indicates receptor staining; gray fill, control staining. ELISA data are represented as mean ± SE of replicate samples.
T-bet negatively regulates cytokine-induced IL-17 production but does not augment cytokine receptor expression on Th1 or Th1β cells. (A) WT or Tbx21-/- Th1 or Th1β cells were cultured for 5 days as in Figure 1A (1° culture). Cells were restimulated with media alone or the indicated cytokines (2° culture) for 18 hours and analyzed for IFN-γ or IL-17 production using ELISA. ND indicates not detected. (B) WT or Tbx21-/- Th1 or Th1β cells were cultured as in Figure 1A. IL-12Rβ2, IL-23R, or IL-18Rα expression was assessed using flow cytometry. Numbers within each histogram represent mean fluorescent intensities averaged from 2 independent experiments. Black line indicates receptor staining; gray fill, control staining. ELISA data are represented as mean ± SE of replicate samples.
Antigen-receptor stimulation induces T-bet expression in Th1β cells, which is required for its progression to an IFN-γ secreting state. (A) WT C57BL/6 Th1 and Th1β cells were cultured for 5 days and left unstimulated or restimulated with α-CD3 with or without 15 μg/mL α-IFN-γ for 4 hours. Protein lysates were analyzed for T-bet expression by immunoblot (B) WT and Tbx21-/- Th1β cells were cultured for 5 days and left unstimulated or were restimulated with α-CD3 for 24 hours. Cell-free supernatants were collected on day 6 and measured for IFN-γ or IL-17 secretion using ELISA. In parallel, 5-day differentiated cells were replated on α-CD3-coated plates and IL-23 for 5 more days as in Figure 1C. After the culture period, live cells were washed and left unstimulated or restimulated with α-CD3 for 24 hours. Cell-free supernatants were collected on day 11. Supernatants from both time points were analyzed for IL-17 and IFN-γ production using ELISA. (C) Tbx21-/- Th1β cells were cultured as in panel B with or without the indicated doses of IFN-γ during the second week of culture. Cells were left unstimulated or were restimulated with α-CD3 for 24 hours, and cell-free supernatants on days 6 and 11 were analyzed for IL-17 production using ELISA. Unstimulated cell populations did not produce detectable cytokine levels (B-C). ELISA data are represented as mean ± SE of replicate samples.
Antigen-receptor stimulation induces T-bet expression in Th1β cells, which is required for its progression to an IFN-γ secreting state. (A) WT C57BL/6 Th1 and Th1β cells were cultured for 5 days and left unstimulated or restimulated with α-CD3 with or without 15 μg/mL α-IFN-γ for 4 hours. Protein lysates were analyzed for T-bet expression by immunoblot (B) WT and Tbx21-/- Th1β cells were cultured for 5 days and left unstimulated or were restimulated with α-CD3 for 24 hours. Cell-free supernatants were collected on day 6 and measured for IFN-γ or IL-17 secretion using ELISA. In parallel, 5-day differentiated cells were replated on α-CD3-coated plates and IL-23 for 5 more days as in Figure 1C. After the culture period, live cells were washed and left unstimulated or restimulated with α-CD3 for 24 hours. Cell-free supernatants were collected on day 11. Supernatants from both time points were analyzed for IL-17 and IFN-γ production using ELISA. (C) Tbx21-/- Th1β cells were cultured as in panel B with or without the indicated doses of IFN-γ during the second week of culture. Cells were left unstimulated or were restimulated with α-CD3 for 24 hours, and cell-free supernatants on days 6 and 11 were analyzed for IL-17 production using ELISA. Unstimulated cell populations did not produce detectable cytokine levels (B-C). ELISA data are represented as mean ± SE of replicate samples.
T-bet regulates IFN-γ expression, and in turn IFN-γ through STAT1 activation promotes expression of T-bet.10,17,18 Despite the reciprocal positive regulation of these genes, T-bet and IFN-γ may also have distinct gene targets that could promote the progression of Th1β cells to the Th1 phenotype described in Figure 1. To distinguish between the direct and indirect actions of T-bet, we tested whether the addition of IFN-γ to T-bet-deficient Th1β cultures for the second week could attenuate IL-17 secretion in a T-bet-independent manner. Tbx21-/- Th1β cells differentiated for 5 days in the absence of exogenous IFN-γ were cultured with the indicated doses of IFN-γ for the second week of culture. Addition of exogenous IFN-γ, at doses up to 600 ng/mL, did not impact IL-17 production by 11-day cultured Tbx21-/- Th1β cells. Together, these data suggest that T-bet expression levels, rather than the presence of IFN-γ, are the critical determinants of Th cell fate.
Discussion
In this report, we have further defined the development of a novel T-helper phenotype that is induced by culture with IL-23. This IL-17-secreting phenotype, which we termed Th1β, and others have termed ThIL-17,9 Th17,7,8 or Tn,19 progresses to a Th1 phenotype following subsequent antigen-receptor stimulation. Importantly, T-bet negatively regulates the Th1β phenotype and facilitates the shift to a Th1 cytokine secretion profile.
The relatedness between Th1 and Th1β cells suggest that they may share expression of a number of genes. Previous mRNA analysis of in vivo-primed cells that were then cultured with IL-12 or IL-23 reported a relatively small number of genes that were differentially expressed between Th1 and Th1β cells.6 However, this experiment was performed with a heterogeneous population of cells and may not reflect gene expression patterns of a more uniform culture. For example, IL-6, which had higher mRNA expression in IL-23-primed cells,6 was not observed in supernatants of Th1β cells (data not shown). In contrast, we do observe the expression of other genes associated with the Th1 phenotype, including the Th1-restricted transcription factors ERM20 and Hlx,21 as well as responsiveness to IL-18 in synergy with IL-12 or IL-23 (Figure 3; data not shown). These data support a close relationship between Th1 and Th1β cells.
Our experiments suggesting a transient nature to the Th1β subset are distinct experiments from those described by Harrington et al.7 In that report, cells cultured for 5 days were stimulated in the presence or absence of skewing cytokines such as IL-4 or IFN-γ, which did not have an effect on the IL-17 levels produced. Our experiments examine cells restimulated following culture for an additional week, a better determinant of the stability and plasticity of T-helper subsets22 and demonstrate that the Th1β phenotype is not stable when antigen receptor engagement occurs. This set of results is clearly different from what has been established for Th1 and Th2 cells.22 This difference may be related to our observation that T-bet levels are dynamically regulated, whereas Th1 or Th2 cells are committed to high or low levels, respectively10 (Figure 4A). It will be interesting to determine whether there is a threshold of T-bet expression required to reverse the phenotype and at what point following T-bet induction cells actually lose their ability to secrete IL-17. In agreement with Harrington et al7 we did not see a demonstrable effect of IL-23 on Th1 or Th2 in vitro-differentiated cells during a second week of culture, in that they were not primed to secrete IL-17. Thus, the Th1β phenotype appears to arise from undifferentiated cells but is not strictly a heritable phenotype.
Although antigen-induced T-bet expression is required for promoting Th1β cultures to an IFN-γ-secreting phenotype, the cellular basis for this progression is not clear. It is possible that IL-17-secreting cells progressively differentiate into IFN-γ-expressing cells following T-bet induction. Alternatively, the development of the IFN-γ-secreting phenotype may be determined by competitive outgrowth of IFN-γ-secreting cells. To distinguish between these possibilities, the isolation of single-positive IL-17 or IFN-γ-secreting T-cell clones and analysis of the kinetics of cytokine secretion patterns and T-bet expression over time is required. T-bet may also play a more direct role in IL-17 regulation. The T-bet-dependent repression of IL-2, IL-21, and the IL-4 gene locus is in part due to T-bet associating with RelA, NFAT, and GATA-3, transactivating factors that are required for the expression of these genes.23-25 Although transcription factors required for mouse IL-17 gene expression have not been completely elucidated, it is possible that T-bet inhibits the function of transcription factors required for IL-17 expression as it does for these other cytokine genes. Future work will define both positive and negative regulators of the IL-17 gene locus.
Recently, Veldhoen et al26 described a role for TGFβ1 and IL-6 in promoting the de novo differentiation of IL-17-secreting cells from naive CD4+ T cells. The investigators suggest that IL-23 is not required for TGFβ1/IL-6-mediated IL-17 production, but rather that IL-23 functions to maintain the IL-17-secreting state from cultures initially primed with TGFβ1 and IL-6. Although it is unclear how TGFβ1 and IL-6 promote IL-17 production, TGFβ1 is known to repress Th1 and Th2 differentiation by inhibiting T-bet and GATA-3 expression, respectively.27-29 In this report, we demonstrate that T-bet is a negative regulator of IL-17 expression, and it is possible that TGFβ1-mediated T-bet repression is critical in TGFβ1/IL-6-induced IL-17 production. If the role of TGFβ1 in the culture system described by Veldhoen et al26 is simply to repress the transcription factors required for Th1 and Th2 differentiation, it would be interesting to determine whether IL-6 is sufficient to promote the IL-17-secreting state from naive T-bet-deficient CD4+ T cells. The relatedness of IL-23 and TGFβ1/IL-6-primed cells, other than their production of IL-17, is still unclear. It is possible that TGFβ1/IL-626 functions on naive cells, whereas IL-23 primarily acts on preactivated cells,5 although these distinctions have not been rigorously defined. Future work will determine whether these 2 populations share other phenotypes and have similar or distinct biologic roles in vivo.
The transient nature of IL-17 secretion and the subsequent default to a Th1 cytokine response following antigen receptor engagement may be essential in modulating immune responses over time in vivo. IL-17 is a potent proinflammatory cytokine that induces granulopoiesis and chemokine expression leading to the rapid recruitment of cells of the innate immune system to inflamed tissues.8,19,30-32 IL-17-mediated responses are required for the eradication of certain microorganisms32-34 but also promote inflammatory diseases.6,9 The continued differentiation of Th1β cells to a Th1 subset following antigen-receptor stimulation could potentially be a mechanism to minimize inflammatory tissue damage. In this context, it is interesting that Th1β cells, in the absence of antigen receptor stimulation, retain the Th1β phenotype (Figure 1) and that maintained antigen levels might actually decrease the inflammatory potential of T-helper cells. This could be mechanistically tied to a requirement for the phenomenon of epitope spreading in chronic autoimmune diseases.35 If Th1β cells are critical for pathology, chronic disease could only be maintained if cells were not repeatedly restimulated or if disease-potentiating Th1β cells can be derived de novo to additional antigens. This suggests several interesting hypotheses to be tested in disease models.
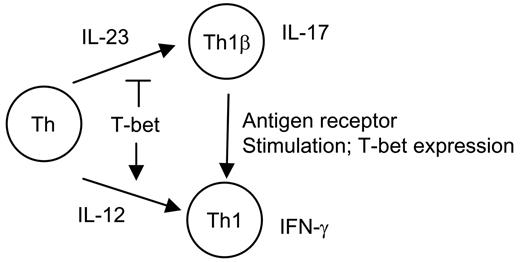
Proposed model for Th1 and Th1β development. CD4+ T-helper cells (Th) in the presence of IL-12 or IL-23 develop into Th1 or Th1β phenotypes, respectively. Although T-bet induces Th1 development, it negatively regulates IL-23-mediated T-cell development. Antigen-receptor engagement of differentiated IL-17-secreting Th1β cells induces T-bet expression and promotes a switch to a Th1 cytokine-secreting phenotype.
Proposed model for Th1 and Th1β development. CD4+ T-helper cells (Th) in the presence of IL-12 or IL-23 develop into Th1 or Th1β phenotypes, respectively. Although T-bet induces Th1 development, it negatively regulates IL-23-mediated T-cell development. Antigen-receptor engagement of differentiated IL-17-secreting Th1β cells induces T-bet expression and promotes a switch to a Th1 cytokine-secreting phenotype.
The lack of autoimmune disease in T-bet-deficient mice, if Th1β cells are more important than Th1 cells in disease development, creates somewhat of a paradox. The IL-23/IL-17 pathway has clearly been shown to be required for the development of inflammatory diseases such as experimental autoimmune encephalomyelitis (EAE) and arthritis.6,9,36 We have shown that T-bet-deficient CD4+ T-cell cultures secrete higher than WT levels of IL-17 and yet T-bet-deficient mice are resistant to the development of EAE.37 This could be a result of several factors, including inherent Th2 skewing of T-bet-deficient CD4+ T cells on activation.12 Th2 cytokines such as IL-10 are essential in negatively regulating inflammatory processes. In addition the Th2 cytokine IL-4 decreases Th1β development.7,8 Therefore, a Th2 cytokine environment may prevent the development of Th1β cells or ameliorate the inflammatory effects of IL-17 in inflammatory diseases. Additionally, T-bet-deficient CD4+ T cells lack critical adhesion molecules and chemoattractant receptors required for migration in inflamed tissues.38,39 It is therefore also possible that T-bet-deficient IL-17-secreting T cells simply fail to migrate to inflammatory foci. However, our studies show that the lack of autoimmunity in the absence of T-bet is not due to an intrinsic inability of CD4+ cells to develop a proinflammatory phenotype.
In summary, we propose the model depicted in Figure 5. Although IL-23 promotes the IL-17-secreting phenotype, this is negatively regulated by the induction of T-bet, which promotes the Th1 phenotype. Restimulation of Th1β through the antigen-receptor results in a switch from the Th1β to the Th1 phenotype. The sensitivity of cell fate decisions to levels of T-bet in the development of Th1 versus Th1β reveal additional complexities of T-cell differentiation and the roles of T-bet in cell-mediated immunity and inflammatory diseases in vivo.
Prepublished online as Blood First Edition Paper, May 2, 2006; DOI 10.1182/blood-2006-04-015016.
Supported by the National Institutes of Health (NIH) Grants AI45515 (M.H.K.), T32DK007519 (H.C.C.), and AI50837 (G.S.K.); and by the Indiana Genomics Initiative of Indiana University (partially supported by the Lilly Endowment) (M.H.K.).
A.N.M. and D.G.Z. are predoctoral fellows of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.