Vascular adhesion protein-1 (VAP-1) is a homodimeric glycoprotein that belongs to a unique subgroup of cell-surface-expressed oxidases. In adults, endothelial VAP-1 supports leukocyte rolling, firm adhesion, and transmigration in both enzyme activity-dependent and enzyme activity-independent manner. Here we studied the induction and function of VAP-1 during human ontogeny. We show that VAP-1 is already found in the smooth muscle at embryonic week 7. There are marked time-dependent switches in VAP-1 expression in the sinusoids of the liver, in the peritubular capillaries of the kidney, in the capillaries of the heart, and in the venules in the lamina propria of the gut. Fetal VAP-1 is dimerized, and it is enzymatically active. VAP-1 in fetal-type venules is able to bind cord blood lymphocytes. Also, adenovirally transfected VAP-1 on human umbilical vein endothelial cells is involved in rolling and firm adhesion of cord blood lymphocytes under conditions of physiologic shear stress. We conclude that VAP-1 is synthesized from early on in human vessels and it is functionally intact already before birth. Thus, VAP-1 may contribute critically to the oxidase activities in utero, and prove important for lymphocyte trafficking during human ontogeny.
Introduction
Leukocyte trafficking between the blood and lymphoid organs is vital for the immune defense. In adults, the extravasation is governed by a multistep adhesion cascade, which involves sequential interactions between endothelial adhesion molecules and their receptors on leukocytes.1,2 One of the endothelial molecules involved in this process is vascular adhesion protein-1 (VAP-1). It is a homodimeric 180-kDa glycoprotein expressed on vascular endothelium.3-5 In vitro binding assays have shown that VAP-1 is needed for leukocyte rolling, firm adhesion, and transmigration during the extravasation cascade in adults.6-8 Animal work using neutralizing anti-VAP-1 antibodies and VAP-1-deficient mice has confirmed its importance in leukocyte trafficking during the physiologic and pathologic conditions in vivo.9-13
VAP-1 belongs to a group of enzymes called semicarbazide sensitive amine oxidases (SSAOs; Enzyme Commission [EC] number 1.4.3.6).14,15 SSAOs catalyze the general reaction R-CH2-NH2 + H2O → R-CH2-CHO + H2O2 + NH3.16,17 In man and mice 3 SSAOs, VAP-1, diamine oxidase, and retina-specific amine oxidase, are known.14,18,19 These amine oxidases are notably distinct from monoamine oxidases A and B in terms of subcellular localization, substrates, inhibitors, cofactors, and function. In fact, leukocyte-endothelial adhesion was the first physiologic function ascribed to SSAOs despite intense research of more than 50 years. Interestingly, this cellular interaction apparently takes advantage of the catalytic nature of VAP-1, since the transient but covalent bond between the enzyme (endothelial VAP-1) and substrate (a surface-expressed amine on leukocyte) is important for leukocyte-endothelial cell interactions under conditions of shear flow.6,15 In addition to endothelium, VAP-1 is expressed in adipocytes, in dendritic cells of germinal centers, and in smooth muscle cells, but not in other types of muscle, in adults.4,20 In adipocytes it contributes to the glucose metabolism and fat cell differentiation,21-25 but on other cell types its functions remain unknown. In addition to the transmembrane VAP-1, a soluble form of the molecule circulates in the blood of adults, and it may modulate the adhesive activity of surface-bound VAP-1.26,27
The mechanisms guiding lymphocyte trafficking during fetal development have remained practically unknown. Moreover, nothing is known from the expression or function of any SSAO molecule before birth. Therefore, we studied the induction and function of the membrane-bound and soluble form of VAP-1 during the human ontogeny.
Materials and methods
Fetal tissues and cells
Human tissue samples were collected from aborted fetuses, from autopsies, and from surgical operations. The 7- and 9-week-old embryos were whole mounts. From later time points (11 weeks [n = 3]), 13 weeks [n = 2], 15 weeks [n = 3], 18 weeks [n = 4], 21 weeks [n = 4], 23 weeks [n = 2], 33 weeks [n = 1], and 40 weeks [n = 6]), multiple organs were collected. These included the following lymphatic tissues: spleen, peripheral lymph nodes (PLNs), small and large bowel, thymus, and bone marrow. In addition, the heart, lung, liver, kidney, adrenal, thyroid, pancreas, ovary, skin, brain, umbilical cord and placenta were obtained. The same organs were also obtained from 2-month-old (n = 2), 2-year-old (n = 2), and 7-year-old (n = 1) children, and from adults (n = 4). The low number of samples and irregular and missing time points were due to the difficulty of obtaining fetal and childhood samples. Our fetal samples younger than 20 weeks were from spontaneous or induced abortions performed by the treating obstetricians according to the Finnish law at the Turku University Central Hospital. Other fetal samples were from stillbirths. The samples from children were obtained from deceased children who underwent autopsy due to clinical reasons. All samples were macroscopically and microscopically normal. Both snap-frozen samples and formalin-fixed, paraffin-embedded samples were prepared, if possible. Not all tissues and organs were available from every individual, but all the results reported were ascertained in a minimum of 2 samples from different individuals of the same age.
Cord blood and adult peripheral blood lymphocytes (PBLs) were isolated using density gradient centrifugation over Ficoll. Serum from cord and adult blood were collected according to standard procedures, aliquoted and stored immediately at -70 °C.
The study protocol was approved by the local Ethics Committee and by the National Board of Medicolegal Affairs in Finland.
Immunohistochemistry
Monoclonal antibodies (mAbs) against human VAP-1 (2D10 and TK8-18) and against mouse VAP-1 (TK10-79) have been described.26,28 2D10 and TK8-14 are function-blocking mAbs that only work with frozen sections, whereas TK10-79, which is cross-reactive with human VAP-1, works with paraffin sections. Anti-CD31 mAb was from Dako (Glostrup, Denmark). As negative controls, mAbs (MECA-367 against mouse MAdCAM-1 and 3G6 against chicken T cells) not reactive with human antigens, and a binding, but not adhesion-blocking mAb, HB151, against human leukocyte antigen (HLA) class I were used.8,29,30
Staining of acetone fixed frozen sections was done using indirect immunoperoxidase staining as described.3 Staining of paraffin sections was done using microwave treatment in a citrate buffer for antigen retrieval and subsequent staining with the avidin-peroxidase method using Vectastain kits (Vector Laboratories, Burlingame, CA) as described.28 All samples were stained for VAP-1, CD31, and negative control. Images were obtained using an Olympus BX60 microscope equipped with a UPlanF1 20×/0.50 numeric aperture [NA] Ph1 or UPlanF1 40×/0.75 NA Ph2 objective. Images were acquired using a ColorView 12 camera (Olympus Soft Imaging Solutions, Münster, Germany).
Biochemical analyses
The molecular weight of VAP-1 in tissues was determined by immunoblotting. Tissue fragments were lysed in 1% NP-40-containing buffer, resolved in SDS-PAGE electrophoresis, and subjected to immunoblotting using ECL detection exactly as described earlier.5
Depletion of VAP-1 from the lysates or serum samples was performed by 3 sequential incubations with cyanogen-bromide-activated Sepharose 4CL beads to which anti-VAP-1 or control mAb were coupled according to a previously described protocol.27
Adhesion assays
The Stamper-Woodruff assay was done as reported.31 In brief, frozen sections of PLNs, intestines, and livers were pretreated with anti-VAP-1 and control mAbs (100 μg/mL), and lymphocytes in suspension were allowed to bind for 30 minutes under constant rotation at 7°C. The results are expressed as a percentage of maximal binding, which is defined by the number of lymphocytes adherent per vessel in control mAb-treated sections. At least 100 vessels were analyzed for each experimental condition in each experiment.
To mimic physiologic blood flow, adhesion assays were also performed using in vitro capillary flow assays with laminar shear stress using a previously described protocol.8,32 In brief, human umbilical vein endothelial cells (HUVECs) were infected with adenoviral construct encoding for VAP-1, grown into confluency, and stimulated with tumor necrosis factor-α (TNF-α; 100 U/mL for 4 hours). The monolayer was then treated with anti-VAP-1 and control mAbs (10 μg/ml) or with an SSAO inhibitor (5 μM hydroxylamine) for 20 minutes. Peripheral blood mononuclear cells (PBMCs) were suspended in RPMI1640 containing 0.1% bovine serum albumin (BSA) and 5 μM histamine and drawn over the endothelial monolayer using a computer-controlled pump and laminar shear stress of 1.0 dyn/cm2 at room temperature. For analysis of rolling and firm adhesion, the cells were allowed to interact for 5 minutes under the shear. For the transmigration assay, the cells were perfused for 10 minutes. Thereafter, the cells were allowed to transmigrate for additional 20 minutes in the presence of continuous flow (without lymphocytes in the flow buffer). After an intial equilibrization period, the interaction of leukocytes with the endothelial cells was observed for 5 minutes (10 predetermined fields, 30 seconds each in rolling/firm adhesion assays and 15 fields in the transmigration assays) under an Olympus IX70 inverted camera (Olympus Optical, Hamburg, Germany) equipped with a Hamamatsu C5405-01 charge-coupled device (CCD) camera (Hamamatsu Photonics, Hamamatsu, Japan). Objectives used included an HMC 10× Plan UIS (NA 0.25; Modulation Optics, Greenvale, NY) and a UPlan FL 10×/0.3 NA (Olympus Optical), along with an IX-LWVCD (NA 0.55) condenser (Olympus Optical). Imaging medium was RPMI 1640 containing 0.1% BSA. Image acquisition software was AnalySIS (Olympus Soft Imaging Systems, Münster, Germany).
Leukocyte-endothelial interactions were then determined offline, as described.8,32 The number of interacting cells observed in the presence of control treatment (anti-HLA-I mAb or vehicle, as appropriate) was defined as 100%. In the rolling/adhesion assays the mean absolute number of analyzed rolling cells per 1 experiment (n = 5 independent experiments using different HUVECs and cord blood cells) was 74 (corresponding to 24 cells/mm2), and that of firmly adherent cells 646 (corresponding to 210 cells/mm2) in the control capillaries. In the transmigration assays, on average 618 adherent and 62 transmigrated cells (corresponding to 134 and 13 cells/mm2, respectively) were scored in each control capillaries (ie, on average 10% of the interacting cells transmigrated in the control capillaries).
Enzymatic assays
The oxidative activity of VAP-1 was measured using a fluoropolarimetric assay, which measures the hydrogen peroxide production in the SSAO-catalyzed reaction, as described.6
Soluble VAP-1 ELISA
The amount of soluble VAP-1 in serum samples was determined using a sandwich enzyme-linked immunosorbent assay (ELISA), exactly as described.26
Statistical analyses
The effect of an anti-VAP-1 antibody or SSAO enzyme inhibitor treatment was compared with the control samples (treated with a control mAb or vehichle, as appropriate) using Student t test. Significance was set at a P value less than .05.
Results
Induction of VAP-1 in specific vascular beds early during fetal development in humans
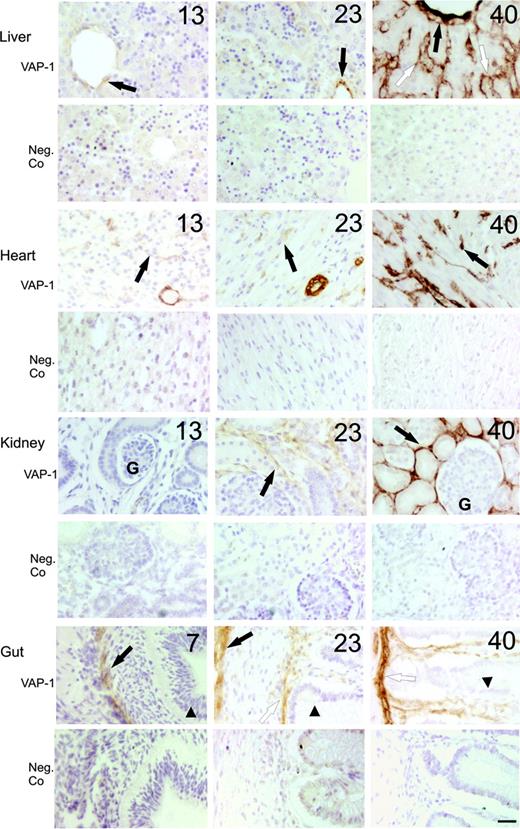
Faint expression of VAP-1 was seen already in our earliest human samples from embryonic week 7. At that stage, VAP-1 expression was confined to the smooth muscle layer of the aortic and gut wall (Figure 1). Thereafter, VAP-1 synthesis appeared in smooth muscle and fat cells in different organs. Notably, skeletal or cardiac muscle cells lacked VAP-1 at all developmental stages.
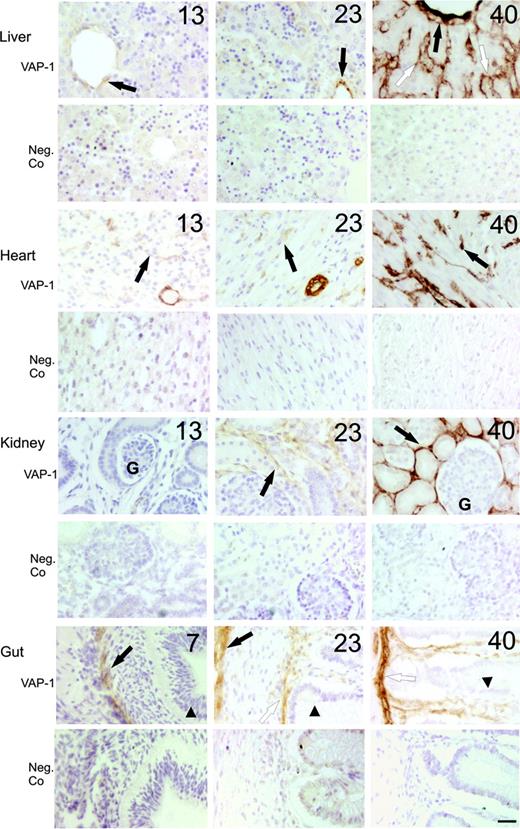
In certain organs developmental switches in VAP-1 synthesis were evident. In the liver, the central vein and smooth muscle cells in the portal tract were faintly VAP-1 positive from 11 weeks onwards. However, the sinusoidal endothelium first showed VAP-1 expression at week 18, and the level of VAP-1 positivity in these vessels only reached adult levels at birth (Figure 1). In heart, VAP-1-positive smooth muscle cells were seen from 11 weeks onwards in the large vessels. Nevertheless, the small capillaries were initially devoid of VAP-1. During the second and third trimesters, a very prominent synthesis of VAP-1 was turned on in these small size vessels (Figure 1). Kidneys remained VAP-1 negative until week 13. Thereafter, occasional VAP-1-expressing smooth muscle cells appeared. It was not before week 23, however, when the peritubular capillaries started to express VAP-1 (Figure 1). Notably, the glomerular endothelium remained VAP-1 negative throughout the life. The lamina propria of intestine was the fourth place where distinct wave of VAP-1 expression was evident. Thus, in the maturing gut, the smooth muscle cells (first in the gut wall from week 7 onwards, and then in large vessels and muscularis mucosae from week 13 onwards) became VAP-1 positive. In the villae, in contrast, VAP-1 was absent from the vessels until week 18, although CD31-positive vessels were clearly observed already at week 9 (Figure 1 and data not shown). Thus, small caliber vessels in the liver, heart, kidney, and gut, which support VAP-1-dependent leukocyte recruitment during adult inflammatory pathologies,4,33-35 become VAP-1 positive gradually and during different time points of fetal development.
Time-dependent induction of VAP-1 in parenchymal organs during fetal development in man. Representative immunohistochemical stainings of VAP-1 in liver (black arrow indicates central vein; white arrow, sinusoid), heart (black arrow, capillary), kidney (black arrow, intertubular capillaries; G indicates glomerulus) and gut (black arrow, gut wall; white arrow, muscularis mucosae; arrowhead, epithelium) at the indicated weeks of gestation are shown. Negative control (Neg.Co) stainings from the same specimens are also shown (lower row for each organ). Scale bar indicates 50 μm. Objective magnification for all images, 20×.
Time-dependent induction of VAP-1 in parenchymal organs during fetal development in man. Representative immunohistochemical stainings of VAP-1 in liver (black arrow indicates central vein; white arrow, sinusoid), heart (black arrow, capillary), kidney (black arrow, intertubular capillaries; G indicates glomerulus) and gut (black arrow, gut wall; white arrow, muscularis mucosae; arrowhead, epithelium) at the indicated weeks of gestation are shown. Negative control (Neg.Co) stainings from the same specimens are also shown (lower row for each organ). Scale bar indicates 50 μm. Objective magnification for all images, 20×.
Expression of VAP-1 in lymphoid organs during development
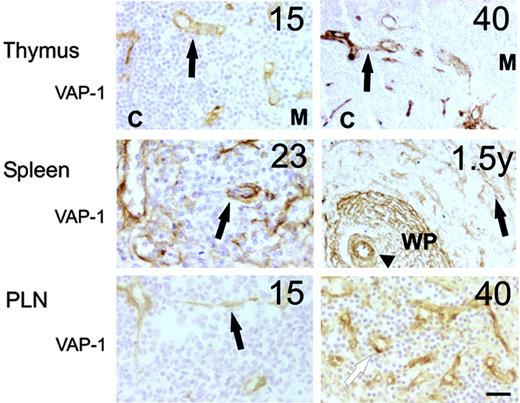
In the primary lymphoid organs very prominent VAP-1 synthesis was seen in thymus during the fetal life. In particular, the cortical preponderance of VAP-1 positivity was notable (Figure 2). In bone marrow, a few vascular sinuses reacted with the anti-VAP-1 mAb, whereas all other cellular elements lacked this molecule (data not shown).
In the secondary lymphoid organs, the spleen displayed strikingly bright VAP-1 expression from early on. Of special interest was to note that the marginal zone sinuses at the margins of white pulp were VAP-1 positive (Figure 2). It is believed that leukocyte entry to the spleen takes place mainly via these channels lined by macrophage-like cells.36 In addition, the dendritic cell-like stromal component in the splenic follicles synthesized high levels of VAP-1 (Figure 2). VAP-1 was present also in high endothelial venules in PLNs already in the earliest samples (15 weeks) we managed to collect (Figure 2).
Nonvascular expression of VAP-1
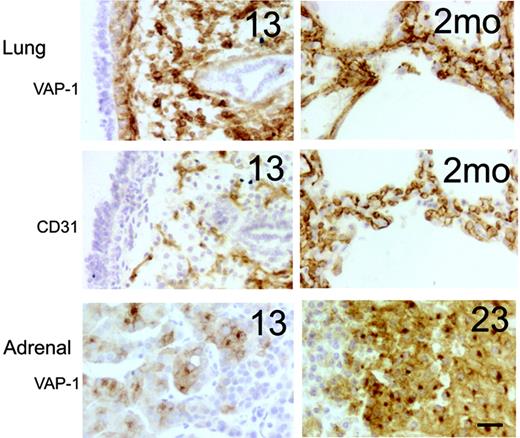
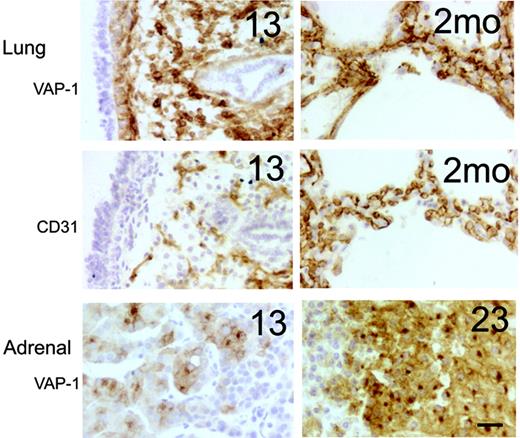
In the developing lung, strong VAP-1 positivity was observed in the stromal cells in the first samples that we had (13 weeks). Comparisons with CD31 expression (an endothelial marker) revealed that VAP-1 was prominently present also on nonendothelial cells (Figure 3). By week 23, the VAP-1 expression became more restricted to the vessel wall (including the smooth muscle cells) in the interalveolar septae, but it did not fully colocalize with CD31-positive endothelial cells, even after birth. In adrenal, VAP-1 positivity was found in the endocrine cells in the cortex already at week 13 (our earliest adrenal specimen), and the staining became more intense at later time points (Figure 3). Notably, the hormone-synthesizing cells lack VAP-1 in the subcapsular granular layer, but they acquired VAP-1 expression at the deeper cell layers. In medulla, again, only the vascular cells showed VAP-1 reactivity.
The expression of VAP-1 in lymphoid organs during the ontogeny in humans. Representative immunohistochemical stainings of thymus (black arrow indicates vessel; C, cortex; M, medulla), spleen (black arrow, vessel; arrowhead, central arteriole; WP, white pulp) and PLN (black arrow, venule; white arrow, high endothelial venule) are shown. The gestational age (weeks, except the right-hand panel of a spleen that is 1.5 years) is shown in the top right corner. Scale bar indicates 50 μm. Objective magnification for all images, 20×.
The expression of VAP-1 in lymphoid organs during the ontogeny in humans. Representative immunohistochemical stainings of thymus (black arrow indicates vessel; C, cortex; M, medulla), spleen (black arrow, vessel; arrowhead, central arteriole; WP, white pulp) and PLN (black arrow, venule; white arrow, high endothelial venule) are shown. The gestational age (weeks, except the right-hand panel of a spleen that is 1.5 years) is shown in the top right corner. Scale bar indicates 50 μm. Objective magnification for all images, 20×.
Prominent synthesis of VAP-1 in embryonic lung and adrenal gland. Representative immunohistochemical stainings from fetal (13 weeks) and postnatal (2 months) lungs and fetal adrenal glands (13 and 23 weeks) are shown. Adjacent sections of the lungs were stained for VAP-1 and CD31 (an endothelial marker). Scale bar indicates 50 μm. Objective magnification for all images, 20×.
Prominent synthesis of VAP-1 in embryonic lung and adrenal gland. Representative immunohistochemical stainings from fetal (13 weeks) and postnatal (2 months) lungs and fetal adrenal glands (13 and 23 weeks) are shown. Adjacent sections of the lungs were stained for VAP-1 and CD31 (an endothelial marker). Scale bar indicates 50 μm. Objective magnification for all images, 20×.
VAP-1 dimers are enzymatically active in fetus
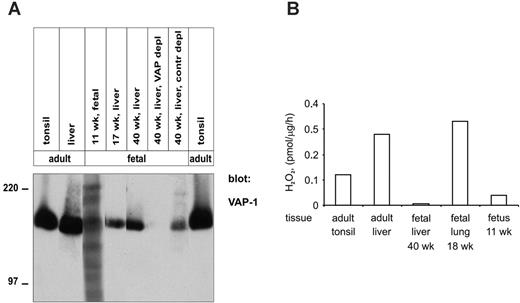
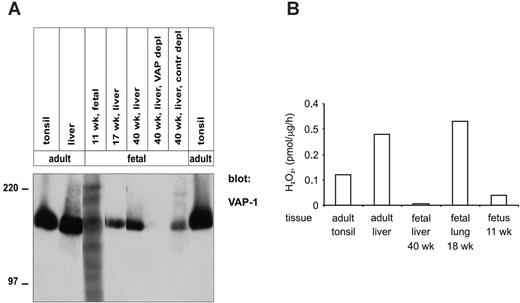
In immunoblotting experiments a faint, specific, approximately 180-kDa band was detected with anti-VAP-1 mAb in a lysate made from a whole 11-week-old fetus. In liver, which mainly contains the endothelial VAP-1, the electrophoretic mobility of VAP-1 in 17- and 40-week-old fetuses was also similar to that seen in adult livers and tonsils (Figure 4A). The specificity of the signal was further confirmed by immunodepleting liver lysate with beads armed with an anti-VAP-1 mAb.
Fetal VAP-1 was able to oxidatively deaminate methylamine, a model substrate for SSAOs. The level of SSAO activity detectable in fetal tissues correlated well to the amount of VAP-1 seen in immunohistochemistry (Figure 4B). Thus, dimerized and enzymatically active VAP-1 is present for at least the 6 last months of the fetal development in man.
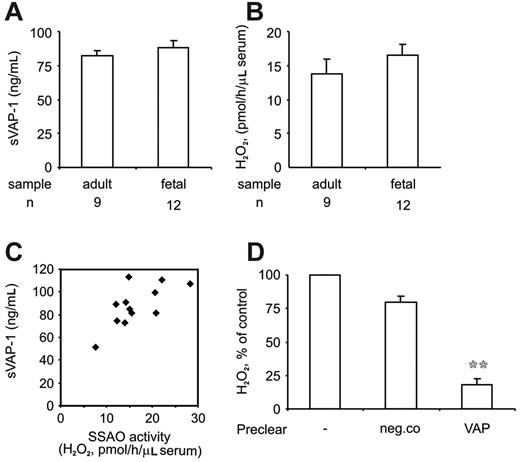
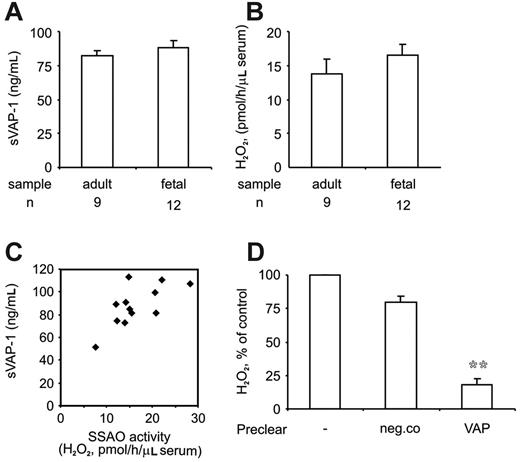
Increased soluble SSAO in fetal blood is derived from VAP-1
The concentrations and activity of soluble VAP-1 (sVAP-1) was determined from sera collected from the cord blood. ELISA assays showed that sVAP-1 protein levels were slightly higher in the cord blood than in the blood circulation of adults (Figure 5A). In the serum collected from the cord blood SSAO enzyme activity was also readily detected (Figure 5B), and again, it was somewhat elevated when compared with the adult levels. There was a good correlation between the sVAP-1 levels and SSAO activity in the cord blood (Figure 5C). sVAP-1 accounted for the majority of SSAO activity, since depletion of sVAP-1 from these samples by sequential immunoprecipitation with anti-VAP-1 mAbs was accompanied with an 80% reduction in the SSAO activity (Figure 5D). Hence, enzymatically active sVAP-1, which may exert regulatory functions in leukocyte adhesion, is present in the blood already before the birth.
Enzymatically active VAP-1 dimers are synthesized already at week 11 of human gestation. (A) Tissue lysates were prepared from the indicated fetal and adult tissues, and analyzed by immunoblotting with anti-VAP-1 mAbs. In addition, the samples from a 40-week liver were precleared with anti-VAP-1 (VAP-1 depl)- or control mAb (contr depl)-armed beads, as indicated. The molecular-weight markers are indicated on the left. (B) Tissue lysates were prepared from the indicated tissues. The specific enzymatic SSAO activity was measured using the fluorometric assay, and is expressed as picomols of H2O2 produced per 1 μg protein in 1 hour. The results from a representative assay, out of 2 with similar results, are shown.
Enzymatically active VAP-1 dimers are synthesized already at week 11 of human gestation. (A) Tissue lysates were prepared from the indicated fetal and adult tissues, and analyzed by immunoblotting with anti-VAP-1 mAbs. In addition, the samples from a 40-week liver were precleared with anti-VAP-1 (VAP-1 depl)- or control mAb (contr depl)-armed beads, as indicated. The molecular-weight markers are indicated on the left. (B) Tissue lysates were prepared from the indicated tissues. The specific enzymatic SSAO activity was measured using the fluorometric assay, and is expressed as picomols of H2O2 produced per 1 μg protein in 1 hour. The results from a representative assay, out of 2 with similar results, are shown.
Increased levels of soluble, enzymatically active VAP-1 in cord blood. (A) The levels of soluble VAP-1 protein (sVAP-1; mean ± SEM) in adult and cord blood were determined using a sandwich ELISA. (B) The SSAO activity of sVAP-1 (mean ± SEM) in sera was determined using the fluorometric assay. (C) The concentration of sVAP-1 was correlated to the SSAO activity in the 12 cord blood samples. (D) VAP-1 is the major SSAO enzyme in cord blood. Serum samples were left untreated or immunodepleted with anti-VAP-1 or control mAb beads, and the remaining SSAO activity (mean ± SEM; n = 3) was determined. **P < .01 between the control mAb and anti-VAP-1 mAb-depleted samples.
Increased levels of soluble, enzymatically active VAP-1 in cord blood. (A) The levels of soluble VAP-1 protein (sVAP-1; mean ± SEM) in adult and cord blood were determined using a sandwich ELISA. (B) The SSAO activity of sVAP-1 (mean ± SEM) in sera was determined using the fluorometric assay. (C) The concentration of sVAP-1 was correlated to the SSAO activity in the 12 cord blood samples. (D) VAP-1 is the major SSAO enzyme in cord blood. Serum samples were left untreated or immunodepleted with anti-VAP-1 or control mAb beads, and the remaining SSAO activity (mean ± SEM; n = 3) was determined. **P < .01 between the control mAb and anti-VAP-1 mAb-depleted samples.
VAP-1 mediates lymphocyte adhesion in newborns
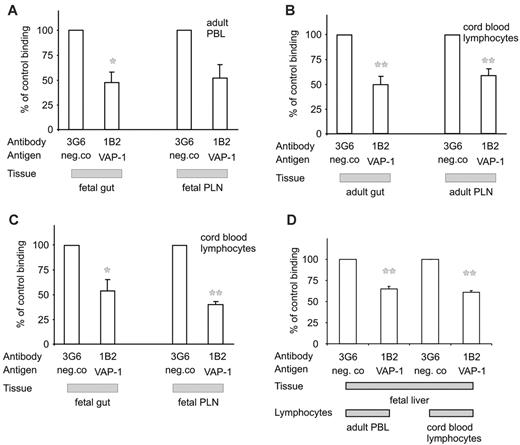
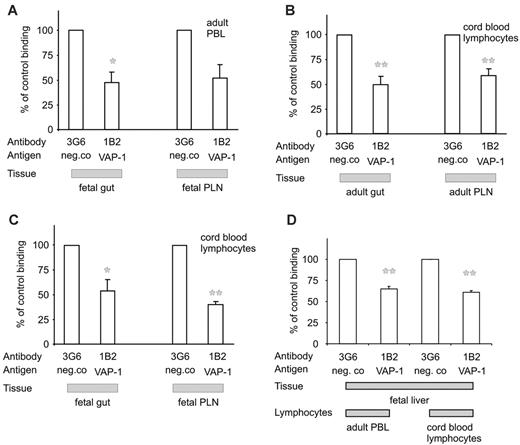
We first analyzed the lymphocyte binding capacity of VAP-1 using a frozen-section binding assay, which reflects well the specificity and molecular mechanisms of leukocyte-endothelial interactions in vivo.37 Guts from 18-week-old fetuses and guts and PLNs from newborns, in which the expression and function of vascular addressins PNAd and MadCAM-1 is still of fetal type, were used.38 Adult PBLs adhered well to the venules in these tissues. Both in the gut and in PLNs, anti-VAP-1 mAbs abrogated about 50% of adult lymphocyte adhesion to high endothelial venules (HEVs) when compared with a non-function-blocking control mAb (Figure 6A). Thus, VAP-1 is functionally intact as an adhesion molecule already in the fetus.
Fetal VAP-1 supports lymphocyte binding to venules. The binding of adult PBLs (A) and cord blood lymphocytes (B-C) to fetal and adult gut and PLN sections in the presence of anti-VAP-1 or control mAb was determined using the frozen-section binding assay. The results are mean ± SEM of 3 independent assays. (D) Adhesion (mean ± SEM) of adult PBLs and cord blood lymphocytes (both from 2 donors) to vessels in 2 different 40-week livers was determined as in panels A-C. *P < .05; **P < .01.
Fetal VAP-1 supports lymphocyte binding to venules. The binding of adult PBLs (A) and cord blood lymphocytes (B-C) to fetal and adult gut and PLN sections in the presence of anti-VAP-1 or control mAb was determined using the frozen-section binding assay. The results are mean ± SEM of 3 independent assays. (D) Adhesion (mean ± SEM) of adult PBLs and cord blood lymphocytes (both from 2 donors) to vessels in 2 different 40-week livers was determined as in panels A-C. *P < .05; **P < .01.
To see whether lymphocytes in newborns are able to bind to VAP-1, we isolated cord blood lymphocytes. We first tested their binding to mature adult VAP-1. These cells bound well to PLNs and gut HEVs, and in both cases the lymphocyte-endothelial cell interaction was strongly dependent on VAP-1 (Figure 6B).
The binding of cord blood lymphocytes to fetal-type vessels was finally tested. Also in this case, anti-VAP-1 treatment inhibited lymphocyte binding to gut vasculature by about 40%. Even more profound VAP-1 dependence of cord blood lymphocyte binding to HEVs was evident in PLNs (Figure 6C).
At 40 weeks, sinusoidal endothelial cells in the liver expressed high levels of VAP-1 (Figure 1), but enzymatically, it appeared relatively inactive (Figure 4B). Therefore, we tested whether VAP-1 is functionally competent in supporting leukocyte adhesion in these vessels. Using the nonstatic binding assay we saw good binding of both adult PBLs and cord blood lymphocytes to vessels of the liver at week 40. An anti-VAP-1 antibody blocked about 40% of binding in both cases (Figure 6D). In similar assays using the same antibody, a 90% reduction was seen when adult PBLs binding to adult liver was measured,33 suggesting that either the expression or adhesive function of VAP-1 further increases later during the postnatal life concurrently with the appearance of the full enzymatic activity. Together, these data show that VAP-1 can mediate lymphocyte binding to endothelial cells in lymphoid and in nonlymphoid tissues under nonstatic conditions already before birth.
Cord blood lymphocytes adhere to VAP-1 in an enzyme-dependent manner under physiologically relevant laminar shear stress
To mimic the physiologic shear in blood vessels more accurately, we finally used in vitro capillary flow assay for the binding experiments. This assay also allows the testing of the contribution of the enzyme activity of VAP-1 to the adhesion. In this set-up, HUVECs were adenovirally transfected to express VAP-1 on this fetal type of endothelial cell. Thereafter, the endothelial cells were pretreated with anti-VAP-1 mAbs, enzyme inhibitors, or relevant controls, and the lymphocytes were perfused over the endothelial cells at a laminar shear of 1.0 dyn/cm.2
When the PBL-VAP-1 interactions were studied it was evident that cord blood cells interacted with VAP-1-expressing endothelium even better than adult PBLs. On average, cord blood lymphocytes rolled 1.8 times better, firmly adhered 3.3 times better, and transmigrated 2.1 times better than adult PBLs (n = 5-7). As shown in Figure 7A, the number of rolling cord blood cells was statistically significantly reduced (by more than 30%) when the endothelium was pretreated with anti-VAP-1 mAb. The firm adhesion of cord blood cells was also constantly and significantly inhibited when blocking the function of VAP-1 with the specific antibody (Figure 7B-C). It should be noted that a mAb against HLA class I, which stains the cells brightly without interfering with leukocyte adhesion, was used as a control. However, anti-VAP-1 mAb did not markedly alter the transmigration of cord blood lymphocytes through the HUVEC monolayer (Figure 7D). Thus, anti-VAP-1 mAb can diminish cord blood lymphocyte rolling and adhesion to VAP-1-expressing endothelial cells under physiologically relevant shear.
VAP-1 supports rolling and firm adhesion of cord blood lymphocytes under physiologically relevant shear. VAP-1 was transfected into HUVECs, and the binding of cord blood lymphocytes was determined using the flow chamber assay under laminar shear stress of 1 dyn/cm.2 HUVECs were activated with TNF-α, and the numbers of the rolling cells (A), firmly adherent cells (B-C) (both from the rolling/firm adhesion protocol [5-minute perfusion] and from the transmigration protocol [30-minute perfusion]) and transmigrated cells (D) was determined in the presence of an anti-VAP-1 mAb (TK-8-14), an SSAO inhibitor (5 μM hydroxylamine), a binding but noninhibiting control mAb (against HLA class I), or vehicle, as appropriate. The results are mean ± SEM of 5 independent assays using different HUVECs and lymphocytes. *P < .05; **P < .01 (all comparisons between the negative control and the indicated treatment).
VAP-1 supports rolling and firm adhesion of cord blood lymphocytes under physiologically relevant shear. VAP-1 was transfected into HUVECs, and the binding of cord blood lymphocytes was determined using the flow chamber assay under laminar shear stress of 1 dyn/cm.2 HUVECs were activated with TNF-α, and the numbers of the rolling cells (A), firmly adherent cells (B-C) (both from the rolling/firm adhesion protocol [5-minute perfusion] and from the transmigration protocol [30-minute perfusion]) and transmigrated cells (D) was determined in the presence of an anti-VAP-1 mAb (TK-8-14), an SSAO inhibitor (5 μM hydroxylamine), a binding but noninhibiting control mAb (against HLA class I), or vehicle, as appropriate. The results are mean ± SEM of 5 independent assays using different HUVECs and lymphocytes. *P < .05; **P < .01 (all comparisons between the negative control and the indicated treatment).
The prototype SSAO inhibitor hydroxylamine was used to block SSAO activity of VAP-1-expressing HUVECs. At the concentration used, it inhibits SSAO activity to the levels seen in cells transfected with a catalytically inactive VAP-1.8 SSAO inhibition caused a 25% reduction in the number of rolling cells (Figure 7). Interestingly, the SSAO inhibitor treatment had no effect on the firm adhesion or transmigration of cord blood lymphocytes. Together, these data suggest that enzyme activity-dependent and enzyme activity-independent functions of VAP-1 are involved in rolling, whereas only the enzyme activity-independent functions contribute to the firm adhesion, and that VAP-1 does not seem to mediate transmigration of cord blood lymphocytes.
Discussion
We report here the first analysis of expression and function of human VAP-1 during development. The ontogenic regulation of this molecule has not been studied in any animal model either, and in fact, it also represents the first developmental characterization of any member of the SSAO enzyme family. The results show that VAP-1 synthesis occurs from embryonic week 7 onward in many tissues and organs. VAP-1 serves its dualistic function already before birth: it secures lymphocyte binding to venules and it is catalytically active as an SSAO enzyme. Moreover, the enzymatic activity of VAP-1 is important in rolling of cord blood lymphocytes on VAP-1-expressing HUVECs under conditions of physiologically relevant shear conditions.
The mechanisms directing lymphocyte recirculation during the development have remained mysterious. We have reported earlier that the mucosal addressin MAdCAM-1 is induced in lymphatic (and nonlymphatic) tissues at time points (7 weeks) comparable with VAP-1 induction, whereas the peripheral addressin PNAd only appears later in week 15.38 MAdCAM-1 and PNAd are aberrantly expressed in a non-tissue-selective manner during the fetal development, and they are functionally active at least around birth.38,39 Hence, many of the vascular adhesion molecules seem to be induced quite early during the lymphoid development. Hence, it is possible that these molecules guide initial seeding of lymphoid cells into the lymphoid tissue anlage. VAP-1 may also contribute to these homing events. This is suggested by its expression in the thymic cortex and in the spleen in those vessels used by the lymphocytes to colonize these organs.
Our data show experimental evidence that fetal type VAP-1 molecule is functionally intact in terms of adhesive and enzymatic activities. VAP-1 expressed in fetal and neonatal lymphoid organs and on HUVECs mediated cord blood lymphocyte adhesion both in nonstatic frozen-section binding assays and in laminar flow assays. This binding involved the oxidase activity of this cell-surface enzyme inasmuch as SSAO inhibitors also blocked lymphocyte-endothelial interaction in the HUVEC model, in which SSAO activity originates solely from the recombinant VAP-1 molecule. Although due to technical limitations with human tissues we were not able to directly analyze leukocyte-endothelial interactions during earlier development, these data give support for the concept that endothelial VAP-1 is involved in leukocyte trafficking during fetal life.
The biological role of SSAO activity has remained enigmatic. We and others have ascribed a direct role for it in leukocyte rolling, adhesion, and transmigration.6,7,9-13,40 This finding and molecular modeling of VAP-1 (based on the crystal structure41 ) implicated leukocyte cell-surface amines as substrates for SSAO.6 In addition to cell adhesion, SSAO has been suggested to scavenge exogenous amines ingested by the alimentary tract.42 Based on the intestinal induction of VAP-1 in the fetus during pregnancy, VAP-1 becomes optimally positioned in intestinal vessels already before birth, although the physiologic consequences of this possible function remain to be shown. The prominent synthesis of VAP-1 specifically in smooth muscle cells, but not in other muscle cell types, could imply that the activity may be associated with maturation of this particular cell type. VAP-1 is also strongly induced on adipocytes during early fetal development. In vitro, the expression of VAP-1 is induced during in vitro differentiation of rodent fibroblastoid cell lines toward mature adipocytes.21-25 In these cells, the SSAO activity contributes to the glucose uptake via the GLUT4 transporter, inasmuch as inclusion of the sVAP-1 substrate benzylamine (in combination with low-dose vanadate treatment) mimics insulin-stimulated glucose uptake. Role of VAP-1 in adipocyte metabolism may be related to its synthesis in endocrine cells in adrenal glands, in which lipid metabolism is important.
There are 4 SSAO enzymes in man and mice, 1 of which appears to be a pseudogene.14,18,19,43 In fact, the current analyses of VAP-1 are the first ontogenic analyses of any of these enzymes in any species. Based on VAP-1 knockout mice studies VAP-1 is the major, if not the only, SSAO enzyme in many tissues.13 The VAP-1-null animals with a strain 129 background do not have any apparent developmental abnormalities. However, these animals have not been subjected to any structural and functional analyses during their development in utero. Therefore, it remains possible that absence of VAP-1 might cause a mild defect in lymphocyte recirculation under physiologic conditions or an impaired host response upon inflammatory challenge, also in utero. Moreover, the expression of VAP-1 differs between mouse and humans. Most notably, the liver and kidney, which are brightly VAP-1 positive in humans, are practically devoid of VAP-1 in mice.28 Therefore, although the available mouse data argue against a central role for VAP-1 during ontogeny, the possibility (which is impossible to verify experimentally) still remains that it may play a role during human ontogeny. Our results show that the expression of VAP-1 is turned on early and in a time-dependent manner in different organs during human fetal development. Once it appears, VAP-1 seems to be thereafter continuously expressed in the vascular wall during all later stages of development. Finally, our data indicate that VAP-1 confers enzymatic SSAO activity early during development, and that it also has adhesive and enzymatic roles in endothelial cells during intrauterine development in man. VAP-1 is currently being targeted in preclinical and early clinical trials by antibodies and small-molecule inhibitors.44-46 Our data imply that VAP-1 may play a role during fetal development in humans, and therefore they should be kept in mind if anti-VAP-1 therapies are at some point planned for use in fertile women.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-11-4599.
Supported by the Finnish Academy and Sigrid Juselius Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Kalle Alanen for providing the tissue material, Kaisa Koskinen for advice with the flow assay, Ms Pirjo Heinilä and Riikka Sjöroos for expert technical assistance, and Ms Anne Sovikoski-Georgieva for secretarial help.


![Figure 7. VAP-1 supports rolling and firm adhesion of cord blood lymphocytes under physiologically relevant shear. VAP-1 was transfected into HUVECs, and the binding of cord blood lymphocytes was determined using the flow chamber assay under laminar shear stress of 1 dyn/cm.2 HUVECs were activated with TNF-α, and the numbers of the rolling cells (A), firmly adherent cells (B-C) (both from the rolling/firm adhesion protocol [5-minute perfusion] and from the transmigration protocol [30-minute perfusion]) and transmigrated cells (D) was determined in the presence of an anti-VAP-1 mAb (TK-8-14), an SSAO inhibitor (5 μM hydroxylamine), a binding but noninhibiting control mAb (against HLA class I), or vehicle, as appropriate. The results are mean ± SEM of 5 independent assays using different HUVECs and lymphocytes. *P < .05; **P < .01 (all comparisons between the negative control and the indicated treatment).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2005-11-4599/2/m_zh80170600460007.jpeg?Expires=1769195031&Signature=l5J3AF8ddsDZuqXO6JVYfUArokt9qpii2JgVMdAdp-ZP2F4iZw1TPG4TqwyUwGDtR55bZH-wVrcq~2JXyIQR1Y1v-6JJxJDbMwy9kWTYWkORcO92oCLk4tA8qLwRScg91Wu0qTGcIFsgcEVAgdB5OS9bvP~IhS2pZeyYpwLt0lHSEXEa7V3t4VIFm8v0Ifjc~W2eztNm7~KlHf~DCgLr89jT9HXoohD7jUkTGZJDLz512aYUnnV3yrOjxkeo0OS8Jivk0Ux-fDXDu1DxSEWwgd1IVFvVIDcPVmgVDC4u4L3ksW-LQ6QbC~rFCQfyEnOTBmjrTzIqd289bPk8oQr4iQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. VAP-1 supports rolling and firm adhesion of cord blood lymphocytes under physiologically relevant shear. VAP-1 was transfected into HUVECs, and the binding of cord blood lymphocytes was determined using the flow chamber assay under laminar shear stress of 1 dyn/cm.2 HUVECs were activated with TNF-α, and the numbers of the rolling cells (A), firmly adherent cells (B-C) (both from the rolling/firm adhesion protocol [5-minute perfusion] and from the transmigration protocol [30-minute perfusion]) and transmigrated cells (D) was determined in the presence of an anti-VAP-1 mAb (TK-8-14), an SSAO inhibitor (5 μM hydroxylamine), a binding but noninhibiting control mAb (against HLA class I), or vehicle, as appropriate. The results are mean ± SEM of 5 independent assays using different HUVECs and lymphocytes. *P < .05; **P < .01 (all comparisons between the negative control and the indicated treatment).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2005-11-4599/2/m_zh80170600460007.jpeg?Expires=1769195032&Signature=iTqMiXcMxfsv8LBiw2SXl8C4Lijy4331~ysh00r2rmLJOYY-1IUNnxbHck5cx3ZyJHTg3Tzn34c54ePsRSYQuCebizAdSuPF7lmb~l63ybgZff6LV7QBqaEwjYkY-tWUHGlJv0p6fLnuareKK3viY7roBrHbCodI5z7JovAgt8Z0Gb18VdpqqhGgnE0uI7DdBMKxOupIirFPwy8bNAW-a0WdK5h~apA36J2DFHv3AtkkfbB9KLNk2~q968mjP7~CocQYkEae0KdYQzk-KxFmVqahwdtxYUsYCEDkl~CQBytdV-B~BaWvAWI-CSRD0E0~oD4F8o-8vKpmws1e21rhyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)