Abstract
CCR5 is a receptor for several β chemokines and the entry coreceptor used by macrophage-tropic (R5) strains of HIV-1. In addition to supporting viral entry, CCR5 ligation by the HIV-1 envelope glycoprotein 120 (gp120) can activate intracellular signals in macrophages and trigger inflammatory mediator release. Using a combination of in vitro kinase assay, Western blotting for phospho-specific proteins, pharmacologic inhibition, CCR5 knockout (CCR5Δ32) cells, and kinase-specific blocking peptide, we show for the first time that signaling through CCR5 in primary human macrophages is linked to the Src kinase Lyn. Stimulation of human monocyte-derived macrophages with either HIV-1 gp120 or MIP-1β results in the CCR5-mediated activation of Lyn and the concomitant Lyn-dependent activation of the mitogen-activated protein (MAP) kinase ERK-1/2. Furthermore, activation of the CCR5/Lyn/ERK-1/2 pathway is responsible for gp120-triggered production of TNF-α by macrophages, which is believed to contribute to HIV-1 pathogenesis. Thus, Lyn kinase may play an important role both in normal CCR5 function in macrophages and in AIDS pathogenesis in syndromes such as AIDS dementia where HIV-1 gp120 contributes to inappropriate macrophage activation, mediator production, and secondary injury.
Introduction
Chemokine receptors, which belong to the G protein-coupled receptor (GPCR) family, regulate diverse cellular responses including adhesion, migration, growth, and survival1,2 and also serve as coreceptors for HIV-1 infection. After the viral envelope surface glycoprotein 120 (gp120) binds to the principal receptor CD4, structural rearrangements enable it to interact with the chemokine receptor. Further structural changes in the transmembrane subunit gp41 trigger membrane fusion and virus entry. Most primary isolates of HIV-1 use CCR5 as a coreceptor (R5 variants), including virtually all strains responsible for person-to-person transmission, establishment and maintenance of infection, and infection within the central nervous system. Strains that use CXCR4 in addition to (R5X4) or instead of (X4) CCR5 arise late in infection in a minority of individuals. CCR5 is a receptor for the β chemokines MIP-1α, MIP-1β, and RANTES and is expressed on cells of macrophage lineage such as monocytes, macrophages, and microglia, as well as on T cells and a limited number of other cell types.3,4
In vivo, macrophages are a principal target both for β chemokine signaling and for R5 HIV-1, but the pathways by which CCR5 regulates macrophage function are not well defined. We previously showed that engagement of CCR5 on primary human monocyte-derived macrophages (MDMs) by recombinant envelope glycoprotein (gp120) from the R5 strain JRFL results in the phosphorylation of several protein kinases, including the focal adhesion-related kinase Pyk2 and members of the mitogen-activated protein (MAP) kinase family, activation of ion channels, and calcium mobilization.5-7 These responses were similar although not identical to responses triggered by MIP-1β, the most specific of the CCR5 ligands. Importantly, CCR5-mediated activation of macrophages by viral envelope triggers a variety of functional consequences including secretion of proinflammatory cytokines including TNF-α and MCP-1.6-8 Dysregulation of macrophages by viral envelopes, independent of viral infection, is believed to be an important factor for the development of HIV-associated dementia (HAD) in late stage AIDS (for a review, see Kaul et al9 ). In addition, CCR5-mediated activation has been implicated in facilitating postentry events involved in macrophage infection.10,11 Thus, defining CCR5 intracellular signaling pathways in macrophages is important both for understanding its normal function in a principal target cell and for mechanisms of AIDS pathogenesis.
A number of GPCRs have been shown to signal through Src family kinases (SFKs), including several chemokine receptors.12-16 Despite its central role in normal immune function and in HIV infection, it is not known whether CCR5 signals through SFKs. Therefore, we sought to determine if SFKs participate in CCR5 signaling in primary human macrophages in response to both its normal ligand and in response to HIV-1 gp120.
Materials and methods
Isolation and culture of primary human MDMs
Human monocytes were obtained from healthy donors by elutriation,17 which yielded cells that were more than 99% pure as determined by fluorescence-activated cell sorting (FACS) analysis of surface markers CD3, CD19, CD4, and CD14. Cells were incubated in RPMI supplemented with l-glutamine, 1% minimal essential amino acids, M-CSF (100 U/mL), and 10% heat-inactivated fetal bovine serum (FBS; Hyclone, South Logan, UT) and maintained in culture for 6 days to allow differentiation into MDMs. After 6 days the cells were harvested and replated in DMEM supplemented with l-glutamine, 1% minimal essential amino acids, and 10% FBS. Prior to stimulation, cells were placed into serum-free medium overnight to induce a quiescent state. Donors were tested for the CCR5Δ32 deletion allele by polymerase chain reaction (PCR),18 and only donors homozygous for the wild-type allele were used unless otherwise indicated.
Reagents
Recombinant JRFL gp120 (provided by R. Doms, University of Pennsylvania) was produced as previously described in 293T cells infected with gp120-expressing recombinant vaccinia viruses.19 Supernatants were clarified by centrifugation and filtered (0.45 μm pore size). Virus was inactivated (0.1% Triton X-100) and gp120 was purified using Galanthus nivalis lectin-coupled agarose beads (Vector Labs, Burlingame, CA) followed by protein concentration and buffer exchange. Env integrity was confirmed by Western blot with a rabbit polyclonal antibody. Additional gp120 preparations were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (SF162, Bal) and Protein Sciences (CM; Meriden, CT). Macrophage inflammatory protein 1β (MIP-1β) was purchased from PeproTech (Rocky Hill, NJ). The CCR5 blocker M65720,21 was kindly provided by M. Miller (Merck, Whitehorse Station, NJ). The Src inhibitors PP2 and Src kinase inhibitor 1, control compound PP3, and ERK-1/2 inhibitors PD98059 and U0196 were from Calbiochem (San Diego, CA). The Lyn-specific inhibitory peptide KRX-123.302 (sequence N to C terminus, where lower case signifies d-isomer: myristyl-G-L-V-T-Y(di-iodo)-k-K-I-K-(amino-benzoyl)-NH2) targets a unique interaction site within Lyn and has been describe previously, as has the negative control peptide KRX-107.110 (sequence N to C terminus: myristyl-G-V-T-R-E-V-P-F-A).22 Peptides were synthesized by Bachem (King of Prussia, PA), purified by high-performance liquid chromatography (HPLC), and dissolved in DMSO (1% maximum final concentration).
Western blot analysis
Week-old MDMs (1 × 106 cells plated in 12-well plates) were placed into serum-free media for 24 hours, stimulated, and then washed with ice-cold PBS. Cells were lysed in radioimmunoprecipitation (RIPA) buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.5% sodium deoxycholate, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS]) supplemented with Complete Mini Protease Inhibitor Cocktail (Roche Diagnostics, Indianapolis, IN). Whole cell lysates (25 μg total protein) were boiled for 5 minutes in Laemmli buffer, clarified (14 000g for 90 seconds at 4°C), subjected to 8% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. Immunoblotting was conducted using primary rabbit polyclonal antibody specific for phospho-ERK (p44/42; Thr 202/Tyr 204), detected with an antirabbit HRP-conjugated antibody, and visualized using chemiluminescent substrate (Amersham Biosciences, Piscataway, NJ). Membranes were then stripped with Re-Blot Plus (Chemicon International, Temecula, CA) and reprobed with rabbit polyclonal antibodies that detect total ERK-1/2. All antibodies were from Cell Signaling Technology (Beverly, MA). In the indicated experiments, MDMs were pretreated for 1 to 2 hours with kinase inhibitors prior to stimulation, and vehicle-only controls were used for experiments in which inhibitors were dissolved in DMSO.
Src immunoprecipitation and in vitro Lyn kinase assay
Stimulated MDMs were harvested in assay lysis buffer (2.0% NP-40, 10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, supplemented with the Complete Mini Protease Inhibitor Cocktail) and placed on ice. Equal amounts of protein per sample were clarified by centrifugation and incubated with anti-Lyn or anti-Hck antibodies (Upstate Biotechnology, Waltham, MA) for 4 hours at 4°C. Preblocked pansorbin beads were added for 30 minutes and washed twice with lysis buffer and once with 10 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.1 mM sodium vanadate. To assess in vitro kinase activity, immunoprecipitates were washed and then incubated with 25 mM HEPES (pH 7.1), 10 mM MnCl2, 1 μM ATP, and 10 μCi (0.37 MBq) γ-32 ATP (6000 μCi/mmol; DuPont NEN, Wilmington, DE) for 5 to 10 minutes at room temperature as previously described.23 The reaction was terminated by the addition of 2 × Laemmli sample buffer and boiled. Samples were analyzed by 8% SDS-PAGE and visualized by autoradiography on Kodak X AR-5 film with enhancing screen at –70°C. To assess levels of total Lyn and Hck protein, lysates from stimulated samples were retained and subject to Western blot using Lyn- or Hck-specific antibodies.
TNF-α ELISA
MDMs (1 × 105 cells/well in 96-well tissue culture plates) were serum starved overnight and duplicate wells were exposed to gp120 for the indicated time points. TNF-α levels in culture supernatants were determined by using the BD OptEIA Human TNF enzyme-linked immunosorbent assay (ELISA) Kit II (BD Biosciences, San Diego, CA) according to the manufacturer's instructions. For blocking studies, inhibitors were added 1 hour before and maintained during the period of stimulation.
Results
We tested the ability of the CCR5 ligand MIP-1β or recombinant HIV-1 envelope glycoprotein gp120 from an R5 HIV-1 primary isolate (HIV-1/JRFL) to activate the MAP kinase ERK-1/2 in primary human MDMs. Based on our previous studies of ionic currents, Pyk2 activation and TNF-α production elicited in macrophages, we used recombinant gp120 and MIP-1β at 40 nM and 25 nM, respectively.5-7 Both R5 gp120 and MIP-1β resulted in the time-dependent phosphorylation of ERK-1/2 MAP kinase (Figure 1A-B), consistent with prior results.7 For both stimuli, peak ERK-1/2 phosphorylation occurred between 5 and 15 minutes after stimulation and typically began to decrease by 30 minutes (data not shown). Recombinant gp120 from 2 other R5 isolates, Bal and SF-162, also triggered ERK-1/2 activation (data not shown). Of note, heat inactivation (95°C for 15 minutes) of gp120 and MIP-1β abrogated ERK-1/2 phosphorylation, confirming that activation was due to a heat-labile protein and did not result from contamination by a heat-stable substance such as endotoxin.
SFKs regulate signaling by some GPCRs, but no role has been identified for SFKs in CCR5 signaling, nor for any chemokine receptor in primary human macrophages. Therefore, we examined MIP-1β and gp120-stimulated MDMs for activity of two Src kinases, Lyn and Hck, known to be highly expressed in macrophages.12 MDMs were stimulated for 1, 5, or 15 minutes, and lysates were immunoprecipitated with Lyn- and Hck-specific antibodies and subjected to in vitro kinase assay. To confirm total Lyn and Hck protein, retained lysates were subject to Western blot. Stimulation with gp120 (Figure 2A) and with MIP-1β (Figure 2B) resulted in the increased activity of Lyn kinase. MIP-1β typically increased Lyn activity rapidly (1 minute), whereas gp120-stimulated Lyn activation was seen from 1 and 5 minutes after stimulation. For both stimuli, Lyn activity returned to baseline by 15 minutes. In contrast, no increased Hck kinase activity was seen following stimulation, although as expected Hck protein was easily detected in MDMs by Western blot (Figure 2C).
R5 gp120 and MIP-1β activate ERK-1/2 in human macrophages. MDMs were stimulated with (A) R5 gp120 (40 nM) or (B) MIP-1β (25 nM). Cell lysates were harvested at the indicated time points and subjected to Western blot with phospho-specific ERK-1/2 (P-ERK) antibodies. Membranes were stripped and reblotted with antibodies to determine total ERK-1/2 protein (T-ERK). ΔHI indicates protein subjected to heat denaturation.
R5 gp120 and MIP-1β activate ERK-1/2 in human macrophages. MDMs were stimulated with (A) R5 gp120 (40 nM) or (B) MIP-1β (25 nM). Cell lysates were harvested at the indicated time points and subjected to Western blot with phospho-specific ERK-1/2 (P-ERK) antibodies. Membranes were stripped and reblotted with antibodies to determine total ERK-1/2 protein (T-ERK). ΔHI indicates protein subjected to heat denaturation.
Lyn kinase activity is up-regulated by gp120 and MIP-1β in macrophages. MDMs were stimulated with (A,C) R5 gp120 (40 nM) or (B) MIP-1β (25 nM). At the indicated time points cell lysates were immunoprecipitated with antibodies specific for Lyn or Hck kinases and subjected to in vitro kinase assay (KA; top rows). In parallel, retained cell lysates (25 μg) were subjected to Western blotting for total Lyn or Hck protein (WB; bottom rows).
Lyn kinase activity is up-regulated by gp120 and MIP-1β in macrophages. MDMs were stimulated with (A,C) R5 gp120 (40 nM) or (B) MIP-1β (25 nM). At the indicated time points cell lysates were immunoprecipitated with antibodies specific for Lyn or Hck kinases and subjected to in vitro kinase assay (KA; top rows). In parallel, retained cell lysates (25 μg) were subjected to Western blotting for total Lyn or Hck protein (WB; bottom rows).
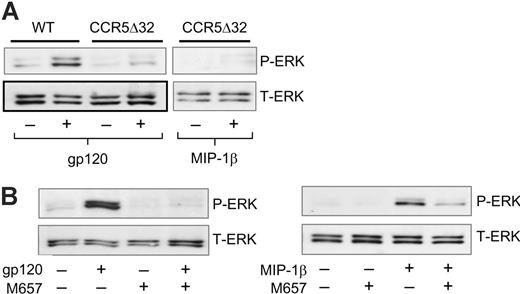
Both MIP-1β and gp120 interact with CCR5, but gp120 first binds to CD4, which triggers the structural rearrangements that enable CCR5 binding. Therefore, we addressed the role of CCR5 in Lyn activation using CCR5 knockout cells. Blood donors were genotyped for the nonfunctional CCR5Δ32 deletion allele and MDMs from homozygous wild-type and CCR5Δ32 individuals were harvested. Cells were stimulated with gp120 or MIP-1β and Lyn kinase activity was examined by in vitro kinase assay (Figure 3). Both MIP-1β and gp120 up-regulated Lyn kinase activity in MDMs expressing CCR5, but neither stimulus up-regulated Lyn activity in cells lacking CCR5. Cell lysates analyzed by Western blot confirmed Lyn expression in both cell types. Thus, Lyn activation by both gp120 and MIP-1β is mediated by CCR5. In this experiment we confirmed that pretreatment for 1 hour with the Src kinase inhibitor PP2 blocks up-regulation of Lyn kinase activity as expected.
Next, we examined the requirement for CCR5 in ERK-1/2 activation using both CCR5Δ32 MDMs and cells pretreated with a CCR5-specific pharmacologic antagonist, M657 (Figure 4). In CCR5-deficient MDMs, neither gp120 nor MIP-1β activated ERK-1/2 (Figure 4A). Similarly, CCR5 blocking by M657 abrogated ERK-1/2 phosphorylation in response to both gp120 and MIP-1β (Figure 4B). Thus, both genetically and pharmacologically based blocking strategies indicate that CCR5 mediates activation of both the Src kinase Lyn and the MAP kinase ERK-1/2 in response to both MIP-1β and HIV-1 gp120.
Lyn kinase activation is CCR5-dependent. MDMs were harvested from donors homozygous for either the CCR5 wild-type (WT) or Δ32 deletion (CCR5Δ32) alleles, pretreated for 1 hour with or without the Src inhibitor PP2 (10 μM), and stimulated for 1 minute with R5 gp120 (40 nM) or MIP-1β (25 nM). Cell lysates were then either immunoprecipitated using anti-Lyn antibodies followed by in vitro kinase assay (KA; top row), or analyzed for total Lyn protein by Western blot (WB; bottom row).
Lyn kinase activation is CCR5-dependent. MDMs were harvested from donors homozygous for either the CCR5 wild-type (WT) or Δ32 deletion (CCR5Δ32) alleles, pretreated for 1 hour with or without the Src inhibitor PP2 (10 μM), and stimulated for 1 minute with R5 gp120 (40 nM) or MIP-1β (25 nM). Cell lysates were then either immunoprecipitated using anti-Lyn antibodies followed by in vitro kinase assay (KA; top row), or analyzed for total Lyn protein by Western blot (WB; bottom row).
CCR5 mediates ERK-1/2 phosphorylation. (A) MDMs from wild-type or CCR5Δ32 homozygous donors were stimulated for 15 minutes with R5 gp120 (40 nM) or MIP-1β (25 nM). Cell lysates were then subjected to Western blot using antibodies specific for phosphorylated (P-ERK) or total ERK-1/2 protein (T-ERK). (B) MDMs were pretreated for 1 hour with the CCR5 antagonist M657 (1 μM), stimulated in the continued presence of the inhibitor with R5 gp120 or MIP-1β, and analyzed by Western blot for phosphorylated or total forms of ERK-1/2.
CCR5 mediates ERK-1/2 phosphorylation. (A) MDMs from wild-type or CCR5Δ32 homozygous donors were stimulated for 15 minutes with R5 gp120 (40 nM) or MIP-1β (25 nM). Cell lysates were then subjected to Western blot using antibodies specific for phosphorylated (P-ERK) or total ERK-1/2 protein (T-ERK). (B) MDMs were pretreated for 1 hour with the CCR5 antagonist M657 (1 μM), stimulated in the continued presence of the inhibitor with R5 gp120 or MIP-1β, and analyzed by Western blot for phosphorylated or total forms of ERK-1/2.
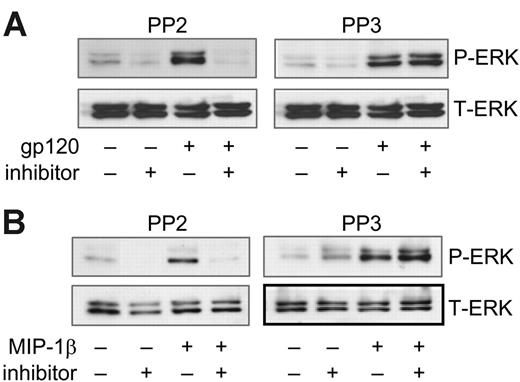
In some cell systems SFKs link chemokine receptors to MAP kinases, but this has not been addressed in primary macrophages or for CCR5. We therefore wished to determine whether SFKs regulate ERK-1/2 activation after CCR5 stimulation in macrophages. MDMs were pretreated with or without the broad Src kinase inhibitor PP2 and then stimulated with gp120 or MIP-1β (Figure 5). PP2 inhibited ERK-1/2 phosphorylation following exposure to both gp120 and MIP-1β (Figure 5A-B left panels). In contrast, the negative control compound PP3 had no effect on ERK-1/2 phosphorylation, (Figure 5A-B right panels). Similar results were seen using another broad Src antagonist, Src-kinase inhibitor I (data not shown).
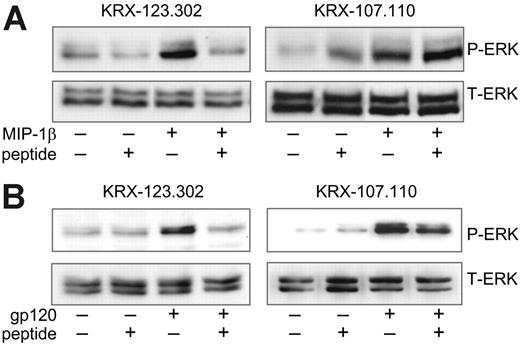
We next determined whether Lyn, which is highly expressed in macrophages and as already demonstrated is activated through CCR5 engagement (Figure 2), is the SFK responsible for linking CCR5 to ERK-1/2. To address this, we used a specific synthetic inhibitor peptide derived from the substrate interaction domain of Lyn, which blocks activation of Lyn but not that of other SFKs.22 MDMs were pretreated for 2 hours with the Lyn-specific KRX-123.302 peptide or with the control peptide KRX-107.110 and then stimulated with MIP-1β (Figure 6A) or gp120 (Figure 6B). The Lyn-specific inhibitory peptide completely abrogated both MIP-1β– and gp120-induced ERK-1/2 phosphorylation, whereas the negative control compound had no effect. Combined with results using PP2, these data indicate that the Src kinase Lyn is a critical link between CCR5 and the MAP kinase ERK-1/2 in primary human macrophages.
ERK-1/2 activation through CCR5 is sensitive to Src inhibition. MDMs were pretreated for 1 hour with the Src kinase inhibitor PP2 (10 μM) or the negative control PP3 (10 μM), then stimulated for 15 minutes in the continued presence of the inhibitor with (A) R5 gp120 (40 nM) or (B) MIP-1β (25 nM). Cell lysates were subjected to ERK-1/2 Western blot using phospho-specific (P-ERK) or total ERK-1/2 antibodies (T-ERK).
ERK-1/2 activation through CCR5 is sensitive to Src inhibition. MDMs were pretreated for 1 hour with the Src kinase inhibitor PP2 (10 μM) or the negative control PP3 (10 μM), then stimulated for 15 minutes in the continued presence of the inhibitor with (A) R5 gp120 (40 nM) or (B) MIP-1β (25 nM). Cell lysates were subjected to ERK-1/2 Western blot using phospho-specific (P-ERK) or total ERK-1/2 antibodies (T-ERK).
Lyn inhibition blocks CCR5-mediated ERK-1/2 activation. MDMs were pretreated for 2 hours with the Lyn kinase peptide inhibitor KRX-123.302 (0.1 μM) or the negative control peptide KRX-107.110 (0.1 μM). Cultures were then stimulated for 15 minutes with (A) MIP-1β (25 nM) or (B) R5 gp120, in the continued presence or absence of peptides. Cell lysates were subjected to ERK-1/2 Western blot using phospho-specific antibodies (P-ERK). Membranes were then stripped and reblotted with total ERK-1/2 antibodies (T-ERK).
Lyn inhibition blocks CCR5-mediated ERK-1/2 activation. MDMs were pretreated for 2 hours with the Lyn kinase peptide inhibitor KRX-123.302 (0.1 μM) or the negative control peptide KRX-107.110 (0.1 μM). Cultures were then stimulated for 15 minutes with (A) MIP-1β (25 nM) or (B) R5 gp120, in the continued presence or absence of peptides. Cell lysates were subjected to ERK-1/2 Western blot using phospho-specific antibodies (P-ERK). Membranes were then stripped and reblotted with total ERK-1/2 antibodies (T-ERK).
Numerous reports have shown that HIV-1 gp120 induces macrophage production of several cytokines or chemokines, which is believed to play a role in inappropriate macrophage activation in AIDS pathogenesis. We and others have recently demonstrated that these macrophage responses are elicited by gp120 interaction with the chemokine receptors.6,7,24 Therefore, we sought to determine whether the gp120-activated CCR5-Src-ERK pathway identified here was involved. We focused on TNF-α because this cytokine is markedly elevated in brains of individuals with AIDS dementia, where it is produced by activated macrophages/microglia and implicated in pathogenesis of this condition.25
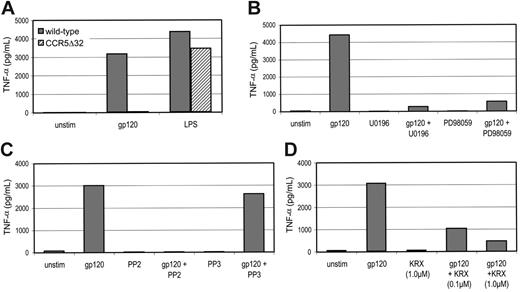
When MDMs from wild-type donors were exposed to gp120, marked increases in TNF-α levels were seen in supernatant 16 hours later (Figure 7). Consistent with reports by others,26 MIP-1β did not elicit TNF-α production (data not shown). MDMs from CCR5Δ32 homozygous donors did not respond to gp120 but did produce TNF-α in response to LPS, confirming the central role of CCR5 in gp120 stimulation (Figure 7A). As expected,7 inhibition of ERK-1/2 blocked TNF-α production in response to gp120 (Figure 7B). We then tested the effect of Src inhibition on TNF-α production in response to gp120 (Figure 7C) and found that the Src inhibitor PP2 completely ablated gp120-induced TNF-α release. We then specifically evaluated the role of Lyn for TNF-α production. MDMs pretreated with the Lyn-inhibitory peptide KRX123.302 at either 0.1 or 1.0 μM demonstrated inhibition of gp120-induced TNF-α production (Figure 7D). These results demonstrate that Lyn, along with ERK-1/2 and CCR5, is critical for this gp120-elicited response. Taken together, these data identify a novel pathway involving CCR5-mediated activation of the Src kinase Lyn and the MAP kinase ERK-1/2 in TNF-α production following macrophage engagement by HIV-1 envelope.
The gp120-triggered macrophage TNF-α production depends on Src, ERK-1/2 and CCR5. (A) MDMs from donors homozygous for CCR5 wild-type or Δ32 alleles were maintained in 96-well plates, stimulated with R5 gp120 (40 nM), or LPS (10 ng/mL), and TNF-α levels in supernatant measured 16 hours later by ELISA. (B-D) MDMs from CCR5 wild-type donors were pretreated for 1 hour with the Src inhibitor PP2 or control compound PP3 (each at 10 μM), the ERK-1/2 inhibitors PD98059 or U0196 (each at 10 μM), or the Lyn-specific inhibitory peptide KRX-123.302 (at either 0.1 or 1.0 μM). They were then exposed to R5 gp120 in the continued presence of the inhibitor, and TNF-α levels in supernatant were measured 16 hours later.
The gp120-triggered macrophage TNF-α production depends on Src, ERK-1/2 and CCR5. (A) MDMs from donors homozygous for CCR5 wild-type or Δ32 alleles were maintained in 96-well plates, stimulated with R5 gp120 (40 nM), or LPS (10 ng/mL), and TNF-α levels in supernatant measured 16 hours later by ELISA. (B-D) MDMs from CCR5 wild-type donors were pretreated for 1 hour with the Src inhibitor PP2 or control compound PP3 (each at 10 μM), the ERK-1/2 inhibitors PD98059 or U0196 (each at 10 μM), or the Lyn-specific inhibitory peptide KRX-123.302 (at either 0.1 or 1.0 μM). They were then exposed to R5 gp120 in the continued presence of the inhibitor, and TNF-α levels in supernatant were measured 16 hours later.
Discussion
CCR5 serves as the receptor for β chemokines MIP-1α, MIP-1β, and RANTES, with MIP-1β the most specific for this receptor. Understanding how CCR5 can signal in macrophages is important for unraveling pathways of chemokine receptor signal transduction and inflammation in a critical primary cell type. It is also important because CCR5 plays a unique and central role in HIV-1 pathogenesis in that it is the coreceptor for most primary isolates of HIV-1 and because viral Env-triggered CCR5-mediated macrophage activation is thought to be an important mechanism of indirect injury in AIDS. This study has identified a novel linkage for CCR5 in primary macrophages that involves the Src kinase Lyn, is activated by both its natural ligand and by the HIV-1 envelope glycoprotein, and links CCR5 to downstream signaling molecules ERK-1/2. This is the first evidence of SFK involvement in CCR5 function and the first demonstration that SFKs mediate chemokine receptor signaling in primary macrophages.
In T cells or CCR5-transfected cells, stimulation by CCR5 natural ligands elevates Ca2+ and activates signals through K+ channels27 ; SHP1, SHP2, and Syk28 ; Pyk229 ; and PI-3 kinase, Jak/STAT, and MAP kinases.30 However, GPCR signaling pathways may be highly cell specific. In primary human macrophages CCR5 stimulation by its natural ligands results in K+ and Cl– ionic currents, Ca2+ elevations, and activation of Pyk2, PI-3, and MAP kinases.5-7 In both T cells and macrophages gp120 stimulation results in CCR5-mediated signals that resemble in some, but not all, respects those elicited by the natural ligands.5-7,29,31,32
Classic GPCR signaling pathways were originally considered distinct from receptor and nonreceptor protein tyrosine kinase pathways, but it is increasingly evident that there is extensive cross-talk between them and, furthermore, that the specific interactions differ markedly in different cell types.33,34 Lyn is one of the predominant SFKs in hematopoietic cells and plays a prominent role in B-cell function where it has both positive and negative regulatory effects on development and in B-cell receptor signaling.35 In macrophages, Lyn is a critical mediator in Fc receptor signaling, a role that it also serves in mast cells, platelets, and other hematopoietic cell types.36 Although Lyn has not previously been linked to CCR5, it is known to be involved in GPCR signaling by the m1 muscarinic acetylcholine receptor,37 the N-formyl peptide chemoattractant receptor in neutrophils,15 and CXCR4 in stem/progenitor cells.23,38
How Lyn is linked in macrophages to CCR5 upstream and ERK-1/2 downstream remains to be elucidated. SFKs may couple GPCR to MAP kinases through several different mechanisms including directly via SH3 domains, through cross-talk with SFK-linked receptor tyrosine kinases, and through both Gα and Gβγ pathways. SFKs may also link GPCR to MAP kinases through complexes with focal adhesion-related kinases (FAK, Pyk2), leading to PI-3 kinase activation.34 In primary macrophages both MIP-1β and R5 gp120 activate Pyk2 and PI-3 kinase, and so it is intriguing to speculate whether Lyn/Pyk2 complexes are involved in this response. Studies are currently underway to determine the relationship between Lyn, Pyk2, PI-3 kinases, and MAP kinases.
Although R5 gp120 and MIP-1β elicit similar signals, there are several differences. We previously showed in macrophages that both chemokines and gp120 activate K+, Cl–, and Ca2+ currents, whereas only gp120 activated a nonselective cation current through CCR5.5 Misse et al reported that in glioma cells both R5 gp120 and MIP-1β activated p38 and JNK MAP kinases, but only MIP-1β activated ERK-1/2.39 In contrast, Madani et al reported that gp120 was a CCR5 antagonist, rather than agonist, in transfected cells.27 Of note, whereas we found that both MIP-1β and R5 gp120 increased Lyn kinase activity and ERK-1/2 phosphorylation, only gp120 induced TNF-α production (Figure 7 and data not shown). The inability of MIP-1β to elicit TNF-α production has been described in murine macrophages, where MIP-1α but not MIP-1β induced TNF-α production despite apparently similar signaling responses.26 The basis for the dichotomous response in that study and for the difference between MIP-1β and gp120 responses seen here is unclear. One obvious difference is that gp120 binds both CCR5 and CD4. However, we found that gp120 stimulation occurs through CCR5 and not CD4, because neither Lyn activation nor other responses to gp120 are seen in CCR5-deficient cells or if CCR5 is blocked pharmacologically, neither of which would prevent the interactions with CD4 that precede chemokine receptor binding. In T cells CD4 is linked to the SFK Lck, which is activated both in normal immune interactions and by gp120,40 but macrophages lack Lck (Pelchen-Matthews et al41 and data not shown) and the normal function and downstream signals linked to CD4 in macrophages is uncertain. Of note, we found macrophage signaling responses to whole virus similar to recombinant gp120 (data not shown), which is consistent with previous reports in dendritic cells, T cells, and several other cell types,42-44 although studies are currently under way to confirm that virion responses are specifically due to gp120 and not virion-incorporated host proteins or other factors.
Although CCR5-elicited macrophage signaling pathways are relevant to mechanisms of immune activation in general, they are particularly important in HIV pathogenesis. Macrophages are a critical target for HIV-1 infection in vivo and although coreceptor signaling is dispensable for infection in transfected cells,45 evidence suggests that signals elicited by gp120 through CCR5 may contribute to early postfusion events necessary for permissive infection of macrophages.10,11 These pathways are also relevant to HAD, a devastating complication of HIV infection that is characterized by widespread activation in brain of both uninfected and infected macrophages/microglia, with consequent production of TNF-α and other proinflammatory and neurotoxic products. The protein and nonprotein products of activated macrophages/microglia that injure neurons in HAD have been the object of intense study (for a review, see Kaul et al9 ), but how HIV activates macrophages/microglia to trigger their release has not been not well defined. Our results support a “bystander effect” mechanism whereby gp120, whether on the surface of noninfectious virion or shed from virions or infected cells, induces specific signaling cascades in uninfected macrophages/microglia resulting aberrant production of mediators such as TNF-α. Moreover, TNF-α is a direct neurotoxin, augments the neurotoxic effect of glutamate and other nonprotein neurotoxins, and inhibits the neuroprotective glutamate uptake by astrocytes (for a review, see Kaul et al9 ). TNF-α production may also activate other cells within the central nervous system to perpetuate and amplify the response, as well as compromise blood-brain barrier permeability. The identification of Lyn as a critical mediator in the gp120-CCR5 macrophage activation pathway helps define the mechanisms involved in “bystander” activation and may help define potential therapeutic targets to block Env-triggered pathologic responses.
Prepublished online as Blood First Edition Paper, April 18, 2006; DOI 10.1182/blood-2005-12-012815.
Supported by National Institutes of Health grants MH061139 and NS027405 (R.G.C.) and CA108552 (A.P.). B.T. was supported by grant AI07324.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Isaacman-Beck for technical assistance; B. Freedman for critical advice and discussions; R. Doms for recombinant gp120 (generated through the NeutralizingAntibody Consortium funded by the InternationalAIDS Vaccine Initiative); and M. Miller for M657. We also thank the Immunology and Virus/Cell/Molecular Cores of the Penn Center for AIDS Research for valuable assistance.