Compared with type I cytokine–associated myeloid (M1) cells, the molecular repertoire and mechanisms underlying functional properties of type II cytokine–associated myeloid (M2) cells are poorly characterized. Moreover, most studies have been limited to in vitro–elicited M2 cells. Here, comparative gene expression profiling of M1 and M2 cells, elicited in murine models of parasitic infections and cancer, yielded a common signature for in vivo–induced M2 populations independent of disease model, mouse strain, and organ source of cells. Some of these genes, such as cadherin-1, selenoprotein P, platelet-activating factor acetylhydrolase, and prosaposin, had not been documented as associated with M2. Overall, the common signature genes provide a molecular basis for a number of documented or suggested properties of M2, including immunomodulation, down-regulation of inflammation, protection against oxidative damage, high capacity for phagocytosis, and tissue repair. Interestingly, several common M2 signature genes encode membrane-associated markers that could be useful for the identification and isolation of M2. Some of these genes were not induced by IL-4/IL-13 or IL-10 under various in vitro settings and thus were missed in approaches based on in vitro–activated cells, validating our choice of in vivo models for expression profiling of myeloid cells.
Introduction
Myeloid cells are characterized by plasticity and versatility in response to microenvironmental signals, resulting in different activation states.1,2 Type I cytokine–associated myeloid (M1) cells, induced by type I proinflammatory stimuli such as IFN-γ, alone or in concert with microbial products such as lipopolysaccharide, produce high levels of reactive nitrogen and oxygen intermediates. Type II cytokines, such as IL-4 and IL-13, antagonize M1 and suppress the production of nitric oxide from l-arginine by inducible nitric oxide synthase. Instead, they induce an alternative metabolic pathway of l-arginine, catalyzed by arginase-1. Factors such as IL-10, glucocorticoids, and transforming growth factor (TGF)–β induce phenotypes that partially overlap with the alternative activation induced by IL-4 and IL-13 and contribute to the heterogeneity of type II cytokine–associated myeloid (M2) cells.3 Hence, some authors have extended the term alternative activation to the effects of these factors.1 Others have proposed to limit this term to the effects of IL-4 and IL-13.2 In this article, we use the term in the strict sense (IL-4 and IL-13 effects) and use the more generic term M2 to describe myeloid cells developed in predominant type II cytokine environments, where cells are affected not only by IL-4 and IL-13 but also by other factors.
Functionally, M2 cells are considered to secure the balance between pro- and anti-inflammatory reactions during type I inflammatory responses and to promote angiogenesis and wound healing.1,3 However, the association of M2 cells with type II inflammatory diseases4 and the ability of M2 cells to induce T-helper 2 differentiation of naive T cells5 suggests that, under these circumstances, M2 cells may contribute to disease.
Compared with M1 cells, M2 cells, especially those elicited in vivo, remain poorly characterized, and reliable markers for M2 are scarce. It is therefore important to obtain a better molecular characterization of in vivo–elicited M2 cells and, in particular, to distinguish them from other types of myeloid cells, such as M1. Such information should provide insight into the specific biologic functions of M2 in vivo and into the molecular basis of these functions. Herein, we aimed to obtain a set of M2-associated marker genes, representing a generic tool for the identification and characterization of M2 populations. We first identified a collection of genes with differential expression in M2 and M1 cells, elicited in an experimental model of trypanosomiasis that has proven useful in the past to discover genes associated with M2.6,7 Next, we analyzed the expression of these genes in various M2 populations elicited in murine models of infectious diseases and cancer and established a common collection of genes overexpressed in M2 compared with M1 cells, approximately half of which had not been previously documented in the context of differential activation of myeloid cells. This common signature for in vivo–elicited M2 transcends the boundaries of in vitro–defined cytokine signatures. Moreover, some of the functional properties of M2 cells can be accounted for by the enhanced expression of common M2 signature genes.
Materials and methods
Parasites, cancer cells, and animals
F1 (C57Bl/6 × BALB/c) and C57Bl/6 mice were inoculated intraperitoneally with phospholipase C–deficient (PLC–/–) Trypanosoma brucei brucei and Trypanosoma congolense Tc13, respectively.8,9 Wild-type, IL-4–deficient,10 or IL-4Rα–deficient11 BALB/c mice were inoculated intraperitoneally with 10 nonbudding Toi strain Taenia crassiceps metacestodes according to published procedures.12 To obtain tumor bearers, AKR mice were inoculated subcutaneously with 2 × 106 BW-Sp3 T lymphoma cells.13 All mice were female and were purchased from Harlan (Zeist, The Netherlands).
Preparation of myeloid cell populations
Peritoneal exudate cells (PECs) from PLC–/–Trypanosoma brucei brucei–infected mice and spleen cells from Trypanosoma congolense–infected animals were collected in the early (2 weeks) and chronic (3-4 months) stages of infection. For the Taenia crassiceps model, PECs were harvested 4 weeks after infection. Spleen cells from BW-Sp3–challenged mice were obtained (5 weeks after injection) from animals with progressing tumors (progressors) or mice that rejected their tumors (regressors). All inoculations were timed so that at the time of cell harvest, mice were age matched. Plastic-adherent peritoneal or splenic myeloid cells were used for expression profiling.6,14 More than 90% of adherent cells were both CD11b+ and F4/80+, as evaluated by cytofluorometry analysis, with fluorescein isothiocyanate (FITC)–conjugated anti-CD11b (BD Biosciences PharMingen, San Diego, CA) and PE-conjugated anti-F4/80 (Serotec, Oxford, United Kingdom) antibodies.6
In vitro stimulation of myeloid cells with cytokines
The adherent population of PECs from noninfected, nontreated BALB/c mice or from mice injected intraperitoneally with 3 mL thioglycollate broth (BioMérieux, Marcy l'Etoile, France) 4 days before cell collection was cultured for 48 hours in the presence of one or all of the following: 100 U/mL IL-4 (BD Biosciences, Franklin Lakes, NJ), 100 U/mL IL-10 (BD Biosciences), 5 ng/mL IL-13 (R&D Systems, Minneapolis, MN). Cell cultures were performed in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 5 × 105 M 2-mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.1 mM nonessential amino acids (all from Invitrogen, Carlsbad, CA).
Differential gene expression analysis
Differential gene expression was analyzed by preparing total RNA using Trizol reagent and performing reverse-transcription using 1 to 2 μg total RNA, 0.5 μg oligo(dT), and 200 U Superscript II reverse transcriptase (all from Invitrogen) in a total volume of 20 μL according to the manufacturer's recommendations, followed by comparison of the intensity of ethidium bromide–stained polymerase chain reaction (PCR) amplicons or by quantitative real-time PCR. For comparative gene expression analysis by ethidium bromide agarose gel electrophoresis, PCR was carried out in a total volume of 50 μL, using 4 μL of (1/8) diluted cDNA reaction as template, 200 nM each dNTP, 0.2 μM each primer, and 1 to 2 U HotStarTaq DNA polymerase (Qiagen, Valencia, CA). To perform comparisons of gene expression within the linear phase of PCR amplification, aliquots were taken from PCR reactions at different cycles, analyzed by agarose gel electrophoresis with 1% agarose gel containing 0.1 μg/mL ethidium bromide, and visualized on a UV transilluminator. Results of the PCR analyses were confirmed in at least 2 independent experiments involving at least 5 mice per condition. Quantitative real-time PCR was carried out using a total reaction volume of 25 μL, containing iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), 4 μL (1:25) diluted cDNA reaction as template, and 0.2 μM each primer in an iCycler (Bio-Rad).7 Each PCR cycle consisted of 1-minute denaturation at 94°C, 45-second annealing at 55°C, and 1-minute extension at 72°C. PCR primer sequences are shown in Table 1. The Ym(1/2) primers amplify both Ym1 and Ym2. The housekeeping gene ribosomal protein S12 (sense primer, GGAAGGCATAGCTGCTGGAGGTGT; antisense primer, CCTCGATGACATCCTTGGCCTGAG) was used as control to ensure that the observed differences in the expression levels of each gene in different cells were not caused by differences in the amount of template cDNA. For quantitative real-time PCR analysis, values were normalized against the housekeeping gene S12.
Detection of protein expression levels in flow cytometry
Fluorescence labeling was performed with the use of CHC (CaCl2/MgCl2-free Hanks balanced salt solution [Invitrogen], supplemented with 40 μM CaCl2 [Merck, Whitehouse Station, NJ]) as staining buffer. Cells were kept in the dark at 4°C throughout all the incubation steps. Cells were first incubated for 30 minutes with purified 2.4G2 anti-CD16/32 antibodies (BD Biosciences PharMingen) to block Fc receptor binding, followed by incubation for 30 minutes with FITC- or PE-bound anti-F4/80 antibodies (Serotec) and washing in CHC. To detect surface expression levels of cadherin-1, FITC-conjugated anti–E-cadherin antibodies or FITC-conjugated control antibodies (all from BD Biosciences PharMingen) were added in parallel to the anti-F4/80 antibodies. Intracellular staining was performed using the Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences), according to the manufacturer's recommendations. Briefly, cells were fixed for 20 minutes in fixation/permeabilization solution, washed in 1 × BD perm/wash buffer (10 × buffer, diluted in CHC), and incubated for 30 minutes with anti–E-cadherin, anti-Sep, or control antibodies diluted in 1 × BD perm/wash buffer. For Sep detection, purified anti-Sep antibodies (BD Biosciences PharMingen) were labeled with PE using the Zenon mouse IgG2b R-PE labeling kit (Invitrogen-Molecular Probes). Positive cells were determined with a FACSVantage station (BD Biosciences, San Jose, CA), and data were analyzed with CellQuest software.
Statistical analysis
Statistical significance was calculated with the unpaired t test and Prism 3.0 software (GraphPad, San Diego, CA). For each experimental setup, results were confirmed in at least 2 independent experiments, each involving at least 3 mice per group.
Results
Identification of genes differentially expressed in M2 compared with M1 cells elicited during PLC–/–Trypanosoma brucei brucei infection
Correlating with a switch in the cytokine environment from type I in the early stage of infection of F1 (C57Bl/6 × BALB/c) mice with PLC–/–Trypanosoma brucei brucei to type II in the chronic phase, myeloid cells from early- and chronic-stage–infected mice are M1 and M2, respectively.8 A subtracted cDNA library, enriched for genes up-regulated in M2 compared with M1, was generated through suppression subtractive hybridization (SSH), using peritoneal macrophages (more than 90% CD11b+ F4/80+) from early- and chronic-stage PLC–/–Trypanosoma brucei brucei–infected mice.6 From this library, 200 clones were randomly selected and sequenced. The identity of each clone was unraveled by homology search against the National Center for Biotechnology Information (NCBI) GenBank DNA sequence databank with the BLAST program (www.ncbi.nlm.nih.gov/BLAST). Approximately 30% of these clones contained fragments of the Ym(1/2) gene, whereas fragments from the other genes were present once or a few times. In total, the 200 clones from the subtracted cDNA library represented 103 unique genes. Based on the sequences obtained from the databank, PCR primers were designed using primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and were checked for their specificities through the BLAST program. Relative expression levels of the 103 genes from the library were first analyzed by PCR on cDNA from differentially activated peritoneal myeloid cells from early- and chronic-stage–infected animals. By comparing the intensities of ethidium bromide–stained PCR amplicons, 30 genes (mentioned in Table 1) were identified whose expression was reproducibly higher in M2 than in M1 cells in the PLC–/–Trypanosoma brucei brucei infection model (ie, it was higher in M2 cells from at least 5 individual mice tested at the chronic stage of infection compared with M1 cells from at least 5 individual mice tested at the early stage of infection). Expression of the other genes from the list of 103 M2-associated genes originally identified in the PLC–/–Trypanosoma brucei brucei model was not higher in at least 60% of M2 populations tested compared with M1 populations. Therefore, these genes were considered false positives of the SSH method and were not retained for the comparisons shown in Table 1. Differential expression of the 30 genes associated with M2 in this infection model was further confirmed using reverse transcription followed by quantitative real-time PCR (Table 1). This analysis revealed that Ym(1/2) mRNA levels were approximately 18 000-fold higher in M2 than in M1 cells elicited during PLC–/–Trypanosoma brucei brucei infection (Table 2). The high redundancy of Ym(1/2) clones in the subtracted cDNA library was likely caused by this high difference in expression, combined with a very high abundance of Ym(1/2) mRNA in M2. In agreement, Ym1/2 mRNA was documented to comprise approximately 10% of the total transcripts of M2 cells elicited in vivo by the nematode Brugia malayi.15
Identification of a common M2 signature by comparing transcript signatures of M2 cells elicited in different disease models
Trypanosoma congolense–infected C57Bl/6 mice mount predominantly type I and predominantly type II immune responses, respectively, associated with M1 and M2 phenotypes of splenic myeloid cells during early and chronic stages of infection.16 Infection of BALB/c mice with the helminth Taenia crassiceps results in gradual induction of a type II cytokine environment and M2.12 In the BW-Sp3 T-cell lymphoma model, subcutaneous inoculation of cancer cells in syngeneic AKR mice results in primary tumors that either regress or progress. Splenic myeloid cells from mice with regressing or progressing tumors are M1 or M2, respectively (Liu et al14 and J.A.V.G., unpublished data, March 2005).
To investigate which of the genes up-regulated in M2 compared with M1 developed during PLC–/–Trypanosoma brucei brucei infection are also overexpressed in M2 cells elicited under other pathologic conditions, their expression was compared in M2 and M1 cells elicited in Trypanosoma congolense–infected or tumor-bearing mice. In addition, because no polarized M1 cells are induced during Taenia crassiceps infection, gene expression levels of M2 from this infection model were compared with those of myeloid cells from noninfected mice. This comparison considered not only differences in disease models and in mouse strains but also in the cell source (peritoneal cavity in the PLC–/–Trypanosoma brucei brucei and Taenia crassiceps models compared with spleen in the Trypanosoma congolense and tumor models). Accordingly, differences were observed in the gene expression patterns of M2 cells elicited in the various models. Yet 13 genes were induced in all M2 populations studied here (ie, in M2 vs M1 in the PLC–/–Trypanosoma brucei brucei, the Trypanosoma congolense, and the BW-Sp3 models and in Taenia crassiceps–elicited M2 vs myeloid cells from noninfected mice), therefore constituting a common signature for in vivo–induced M2 (Tables 1 and 2). In other words, from the 30 M2-associated genes originally identified in the PLC–/–Trypanosoma brucei brucei model, those not up-regulated in M2 in one or more of the other models studied here were excluded from the common M2 signature.
The identified common M2 signature included plasma platelet–activating factor acetylhydrolase (Pafah), prosaposin, selenoprotein P (Sep), and growth arrest–specific gene 3 (Gas3), triggering receptor expressed on myeloid cells 2 (Trem2) and cadherin-1 (E-cadherin) for which an association with M2 has not been previously documented.
Cytokine regulation of M2-associated genes
We investigated whether genes from the common M2 signature were inducible in vitro by IL-4, IL-13, and IL-10, the prototypical cytokines affecting M2 differentiation.1-3 We used plastic-adherent resident, nonelicited, or thioglycollate-elicited peritoneal lavage cells from noninfected BALB/c mice. Compared with nonstimulated cells, gene expression levels of arginase-1, macrophage mannose receptor (Mmr), found in inflammatory zone 1 (Fizz1), macrophage galactose-type C-type lectins 1 and 2 (Mgl1 and Mgl2), Ym(1/2), and cadherin-1 were induced by IL-4 (Table 2) and IL-13 (data not shown) stimulation in resident and thioglycollate-elicited macrophages. Trem2 and folate receptor 2 (Folr2) were induced by IL-4 and IL-13 in resident, but not in thioglycollate-elicited, macrophages. Sep, arginase-1, Mmr, and Folr2 were inducible by IL-10 in resident and thioglycollate-elicited macrophages. We could not detect significant (P < .05), reproducible (ie, in all of at least 3 different experiments) induction of Pafah, prosaposin, and Gas3 gene expression by any of the cytokines under the conditions tested (Table 2).
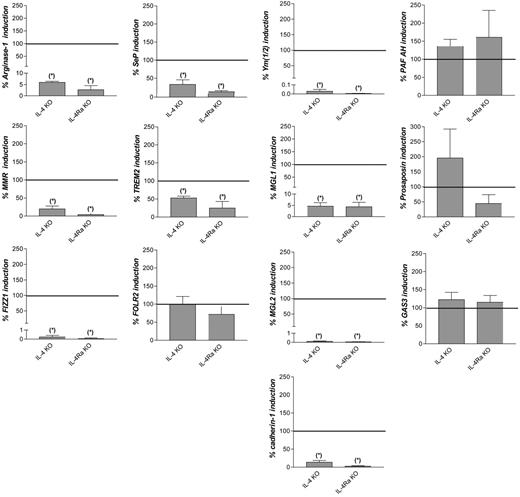
To investigate the contribution of IL-4 and IL-13 signaling to the in vivo induction of the common M2 markers, Taenia crassiceps infections were induced in wild-type (wt), IL-4–deficient (IL-4 KO), and IL-4Rα–deficient (IL-4Rα KO) BALB/c mice. In peritoneal macrophages from noninfected animals, expression levels of the common M2 marker genes were similar among the 3 types of mice (data not shown). In addition, on Taenia crassiceps infection of the 3 types of mice, a similar parasite burden was recorded until peritoneal macrophages were isolated (data not shown). In accordance with a previous report,7 induction of arginase-1, Mmr, Fizz1, Ym(1/2), Mgl1, and Mgl2 was significantly lower in IL-4 and IL-4Rα KO than in wild-type mice during infection (Figure 1). This was also the case for cadherin-1, Sep, and Trem2 but not for Folr2, Pafah, prosaposin, and Gas3.
Protein expression levels of common M2 markers
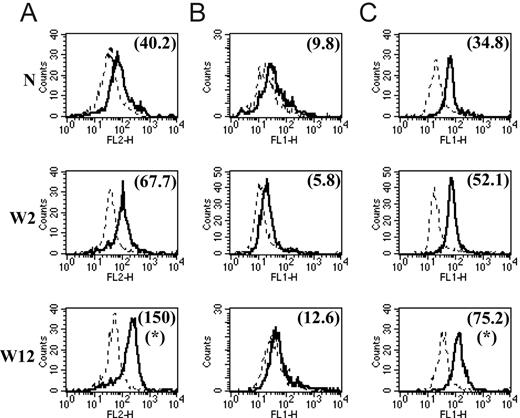
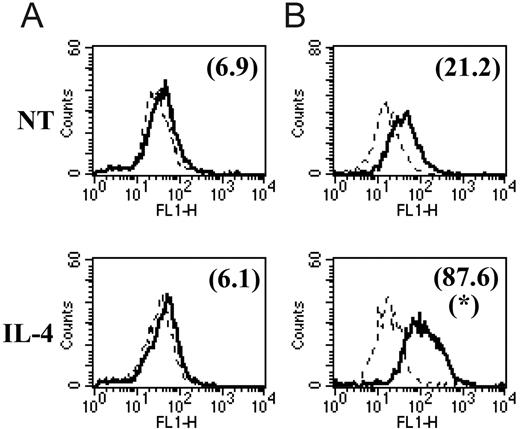
We and others7,17-19 have already reported that the common M2 marker genes MMR, arginase-1, FIZZ1, Ym1, MGL1, and MGL2 are also up-regulated at the protein level in M2 compared with M1. In this study, we focused on the protein expression level of 2 of the newly identified common M2 markers for which tools were available. With the use of intracellular staining and flow cytometry analysis, we could detect significantly augmented Sep and cadherin-1 levels in peritoneal macrophages from chronic-stage PLC–/–Trypanosoma brucei brucei–infected mice (M2) compared with those from mice at the early stage of infection (M1) and compared with noninfected mice (Figure 2A, C). Intriguingly, we could not detect surface expression of cadherin-1 above the background on peritoneal macrophages at different stages of PLC–/–Trypanosoma brucei brucei infection (Figure 2B). In view of the intriguing results obtained in these ex vivo macrophages, we also analyzed the effect of in vitro IL-4 stimulation on cadherin-1 expression levels. In vitro incubation of thioglycollate-elicited macrophages from noninfected mice with IL-4 induced an increased intracellular expression of cadherin-1 but did not result in detectable surface expression (Figure 3) under the conditions tested.
Discussion
Despite their impact on the outcome of various diseases,20,21 M2 cells, especially those elicited in vivo, remain poorly characterized. In the present study, we analyzed gene expression profiles of M2 compared with M1 cells elicited in a number of in vivo murine disease models. In spite of differences in gene expression patterns of M2 cells elicited in the various models, a number of genes were found to be induced in all M2 populations studied here, independent of disease model, mouse strain, or organ source of cells. These genes thus represent a common signature for M2 cells elicited in various in vivo settings characterized by predominant type II cytokine environments, and they constitute a generic toolbox for the identification and characterization of M2 populations.
The common signature included the documented M2-associated genes arginase-1, Mmr, Fizz1, Ym(1/2), Mgl1, and Mgl2,6,7,15,22 thus validating the identified signature genes. These genes, as well as cadherin-1, were inducible by IL-4 and IL-13 in vitro and reflect transcript fingerprints of alternatively activated myeloid cells senso strictu.2 Induction of arginase-1, Mmr, and Folr2 by IL-10 is in accordance with previous reports.23-25 Sep was inducible in vitro by IL-10, but not by IL-4 or IL-13. Yet, during Taenia crassiceps infection, induction of Sep was partially impaired in IL-4 KO and IL-4Rα KO mice compared with wild-type mice. These results suggest an indirect role for IL-4 and IL-13 in Sep induction in vivo. We do not favor the hypothesis that reduced induction of Sep in the IL-4 and IL-4Rα KO mice reflects a synergistic effect of IL-10 with IL-4 or IL-13 in vivo because, in vitro, combined stimulation with IL-10, IL-4, and IL-13 did not induce Sep expression to levels higher than those obtained with IL-10 alone (data not shown). However, we cannot exclude that certain factors, present in vivo but lacking in the experimental in vitro setting, may be required for optimal Sep induction.
Contribution of IL-4 and IL-13 signaling to induction of common M2 marker genes during Taenia crassiceps infection. Fold induction of the genes in peritoneal macrophages from Taenia crassiceps–infected, IL-4–deficient (IL-4 KO) or IL-4Rα–deficient (IL-4Ra KO) BALB/c mice 32 days after infection compared with noninfected mice, expressed as percentage of the fold induction in wild-type mice. Gene expression was determined by reverse transcription followed by real-time quantitative PCR and was normalized for the housekeeping gene ribosomal protein S12. Values indicate the mean of at least 3 mice in each condition. Error bars indicate SEM. Data are shown for 1 representative experiment. *Significantly lower than in infected wild-type mice (P < .05).
Contribution of IL-4 and IL-13 signaling to induction of common M2 marker genes during Taenia crassiceps infection. Fold induction of the genes in peritoneal macrophages from Taenia crassiceps–infected, IL-4–deficient (IL-4 KO) or IL-4Rα–deficient (IL-4Ra KO) BALB/c mice 32 days after infection compared with noninfected mice, expressed as percentage of the fold induction in wild-type mice. Gene expression was determined by reverse transcription followed by real-time quantitative PCR and was normalized for the housekeeping gene ribosomal protein S12. Values indicate the mean of at least 3 mice in each condition. Error bars indicate SEM. Data are shown for 1 representative experiment. *Significantly lower than in infected wild-type mice (P < .05).
Pafah, prosaposin, and Gas3 were not significantly affected by any of these cytokines in the in vitro settings used here, though their expression was induced in all the in vivo M2 populations investigated. Moreover, induction of these genes in macrophages from Taenia crassiceps–infected IL-4 or IL-4Rα KO mice was not significantly impaired compared with induction in wild-type mice. These genes would thus most likely have been missed in approaches based on in vitro–activated cells, validating our strategy of using a range of in vivo models to identify M2-associated genes. Hence, the collection of M2 signature genes identified in this study transcends the boundaries of in vitro–defined cytokine signatures and goes beyond the effects of merely IL-4 and IL-13, but it takes into account other factors to which myeloid cells are exposed in vivo in predominantly type II cytokine environments. Moreover, the finding that IL-4/IL-13 and IL-10 signature genes, as well as genes induced by neither of these cytokines, are among the common M2 signature is in accordance with the recently reviewed heterogeneity of M2 populations, reflecting their plasticity and versatility in response to a complex mixture of microenvironmental signals.3
The ability to analyze the in vivo heterogeneity of M2 depends on the availability of many different markers, in particular surface markers. In this respect, approximately half the genes belonging to the common signature of M2 are potentially membrane associated (Table 2). These markers, alone or in combination, can thus be of particular interest to identify, isolate, and characterize M2.
Enhanced expression at the protein level in M2 compared with M1 had already been documented for a number of genes belonging to the common M2 signature, including Mmr, arginase-1, Fizz1, Ym1, Mgl1, and Mgl2. In the present study, we extended this observation to Sep and cadherin-1, 2 of the newly identified M2 signature genes for which tools were available. In particular, enhanced intracellular expression of cadherin-1 and Sep was found in M2 compared with M1 populations. However, we could not detect cadherin-1 surface expression in the M2 populations investigated in this study. Cadherin-1 surface expression is a dynamic process, dependent on the balance between cadherin-1 exocytosis and endocytosis.26 Accumulation at the cell surface is dependent on physiologic (co)stimuli or on contact with other cells expressing cadherin-1 ligands.26,27 Interesting results were obtained in this context with macrophages isolated from progressing subcutaneous BW-Sp3 tumors. These cells are M2 oriented (ie, they express common M2 marker genes, including cadherin-1, at the mRNA level) and express cadherin-1 at their surfaces (J.A.V.G., unpublished data, March 2005).
Functionally, cadherin-1 is the prototypical epithelial cadherin and is also known as E-cadherin. It is a major component of intercellular junctions and plays a critical role in epithelial morphogenesis and integrity through homophilic interactions.28 On the other hand, by binding to the integrin CD103, cadherin-1 mediates heterotypic adhesive interactions between epithelial cells and intraepithelial lymphocytes, thus contributing to lymphocyte homing and migration to tissues.29 Such interactions of cadherin-1 with CD103 were shown to be necessary for trafficking and retention of regulatory T cells to sites of infection; thus, they were involved in the control of excessive immune responses.30 In immature dendritic cells, cadherin-1 expression is promoted by IL-4 and TGF-β1,31,32 and it has been suggested that they are involved in the interaction of these cells with lymphocytes within mucosal and dermal tissues.33 In addition to its role as an adhesion molecule, cadherin-1 also mediates outside-in signaling into cells, which is dependent on cadherin-1 internalization and trafficking along endocytic pathways.34 To date, a defined functional role for cadherin-1 in macrophages has not been reported. We have initiated experiments to study the function of cadherin-1 expressed on M2-oriented tumor-associated macrophages.
Protein expression levels of Sep and cadherin-1 in peritoneal macrophages from noninfected mice (N) and PLC–/–Trypanosoma brucei brucei–infected mice 2 weeks (W2) and 12 weeks (W12) after infection. Total cells from peritoneal lavage were stained with FITC-labeled anti-F4/80 antibodies (A) and with PE-labeled anti-F4/80 antibodies (B-C). Intracellular Sep expression (A), determined using Zenon-PE labeled anti-Sep antibodies (solid lines). Cadherin-1 surface (B) and intracellular (C) expression, determined using FITC-labeled anti–cadherin-1 antibodies (solid lines). Expression was determined by direct immunofluorescence and flow cytometry analysis. Expression profiles represent the distribution of fluorescent cells in function of fluorescence intensity in the PE channel of the flow cytometer (A) and in the FITC channel (B-C) for gated F4/80-positive macrophages. The dashed line corresponds to the background profile of cells stained with Zenon-PE–labeled control antibodies (A) and FITC-labeled control antibodies (B-C). Numbers between brackets indicate the background-subtracted median fluorescence intensities. Results are shown for 1 of at least 3 representative independent mice for each condition. *Significantly higher than in noninfected and in early stage–infected mice (P < .05).
Protein expression levels of Sep and cadherin-1 in peritoneal macrophages from noninfected mice (N) and PLC–/–Trypanosoma brucei brucei–infected mice 2 weeks (W2) and 12 weeks (W12) after infection. Total cells from peritoneal lavage were stained with FITC-labeled anti-F4/80 antibodies (A) and with PE-labeled anti-F4/80 antibodies (B-C). Intracellular Sep expression (A), determined using Zenon-PE labeled anti-Sep antibodies (solid lines). Cadherin-1 surface (B) and intracellular (C) expression, determined using FITC-labeled anti–cadherin-1 antibodies (solid lines). Expression was determined by direct immunofluorescence and flow cytometry analysis. Expression profiles represent the distribution of fluorescent cells in function of fluorescence intensity in the PE channel of the flow cytometer (A) and in the FITC channel (B-C) for gated F4/80-positive macrophages. The dashed line corresponds to the background profile of cells stained with Zenon-PE–labeled control antibodies (A) and FITC-labeled control antibodies (B-C). Numbers between brackets indicate the background-subtracted median fluorescence intensities. Results are shown for 1 of at least 3 representative independent mice for each condition. *Significantly higher than in noninfected and in early stage–infected mice (P < .05).
In addition, a documented role in macrophages is lacking for Sep, another common M2 signature gene for which we could detect enhanced expression in M2 compared with M1 at the protein level. This extracellular glycoprotein, rich in selenocysteine, is involved in selenium homeostasis. Liver-derived Sep is the major selenium transporter in the serum. In the brain, Sep acts as a local selenium storage and recycling protein essential for maintaining brain selenium levels.35 In addition, Sep seems to function in antioxidant defense. Indeed, it is a phospholipid hydroperoxide glutathione peroxidase,36 acting as a scavenger of free radicals such as peroxynitrite in vitro,37 and it binds to endothelial cells in vivo.38 Its pH-dependent heparin-binding properties suggest that Sep binds to host cell membranes under conditions induced in areas of inflammation.39 Accordingly, Sep plasma levels correlate with the prevention of diquat-induced lipid peroxidation and hepatic endothelial cell injury in rats.40 Moreover, Sep expressed in human pancreatic cancer cells is associated with resistance to the chemotherapeutic agent gemcitabine by suppressing the induction of reactive oxygen species.41 Conversely, Sep serum levels are significantly reduced in patients with inflammatory bowel disease, particularly Crohn disease.42 Because of its enhanced expression in M2 developed during the chronic stages of trypanosome infections, we are analyzing a potential role for Sep in the protection against infection-associated, immune-mediated disease.
Other genes from the common M2 signature can be clustered in a group in which the gene products have documented angiogenic, wound healing, or anti-inflammatory properties (Table 2). This cluster includes the wound healing-promoting enzyme arginase-1, whose up-regulation is a hallmark of alternative activation of myeloid cells,2 and the angiogenesis-promoting factor Fizz1.43 The newly identified common M2 signature gene Pafah also belongs to this functional cluster. This secreted phospholipase A 44 2 is a potent anti-inflammatory agent by virtue of its ability to catalytically inactivate platelet-activating factor and related pro-inflammatory phospholipids.45,46 In vivo LPS administration was documented to induce Pafah expression in resident liver and peritoneal macrophages.47 Finally, we have also included in this cluster the sphingolipid hydrolase activator prosaposin, which reportedly plays a role in attenuating free radical–induced neuronal damage,48 and Trem2, a pattern recognition receptor (PRR) recognizing ligands on bacteria and mammalian cells49 and involved in the augmentation of apoptotic neuron phagocytosis and the attenuation of proinflammatory cytokine secretion by microglia.50
Obviously, many of the M2 signature genes are pleiotropic and may even have effects opposite those mentioned. For instance, cross-linking of surface-expressed Trem2, transfected into macrophages, was shown to result in the release of nitric oxide.51 As another example, excessive or improperly regulated arginase-1 expression can impair wound healing and contribute to disease.52,53 This may reflect functional plasticity or heterogeneity of M2 cells in vivo, depending on the environmental context in which they are operative. Yet overall the association with various M2 populations suggests that these genes contribute to common pathways through which in vivo–elicited M2 cells dampen type I inflammation and promote angiogenesis and tissue repair, functions that have been attributed to M2 cells1 but for which the underlying mechanisms have been poorly understood.
In vitro cytokine modulation of cadherin-1 protein expression levels. Thioglycollate-elicited peritoneal macrophages were incubated in vitro for 48 hours in the presence of IL-4 or in the absence of cytokines (NT indicates no treatment control). Cadherin-1 surface (A) and intracellular (B) expression were determined and are represented as described in the legend to Figure 2. *Significantly higher compared with no treatment (P < .05). Solid and dashed lines are as in Figure 2.
In vitro cytokine modulation of cadherin-1 protein expression levels. Thioglycollate-elicited peritoneal macrophages were incubated in vitro for 48 hours in the presence of IL-4 or in the absence of cytokines (NT indicates no treatment control). Cadherin-1 surface (A) and intracellular (B) expression were determined and are represented as described in the legend to Figure 2. *Significantly higher compared with no treatment (P < .05). Solid and dashed lines are as in Figure 2.
Another common characteristic of M2 revealed here is the up-regulated expression of several lectins. Although the role of PRRs of M1 in the induction of type I immune responses is well characterized, little is known about PRRs of M2. Signaling through C-type lectins such as Bdca-2 and mannose receptor down-regulates type I and induces type II immune responses.54,55 It is therefore possible that M2-expressed lectins represent PRRs, involved in the uptake and clearance of pathogens and skewing the immune system toward a type II response.56,57 A role for lectins in immune modulation is further supported by the recent finding that MGL expressed on human dendritic cells attenuates inflammatory cytokine secretion by T cells through interaction with CD45.58
In conclusion, in the present study, we have identified a common signature for in vivo–elicited M2 cells that should facilitate their identification, characterization, and isolation, thereby allowing better understanding of their roles in different pathologic conditions. Moreover, the documented properties of the products of these common M2 signature genes, of which approximately half had not been documented as associated with M2, provide a molecular basis for a number of documented or suggested properties of M2 cells, including anti-inflammation, immunomodulation, high capacity for phagocytosis, and wound healing.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-04-1485.
Supported by postdoctoral fellowships from the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT-Vlaanderen) (G.R.) and from the Prospective Research for Brussels program (J.A.V.G.); by grants from IWT-Vlaanderen for Generisch Basisonderzoek aan de Universiteiten (IWT-GBOU), Fund for Scientific Research Flanders (FWO-Vlaanderen), and United Nations Children's Fund (UNICEF)/United Nations Development Programme (UNDP)/World Bank/World Health Organization (WHO) Special Programme for Research and Training in Tropical Diseases (TDR); and by a bilateral international scientific and technologic cooperation grant from the ministry of the Flemish community. F.B. is a Wellcome Trust Senior Fellow for Medical Science in South Africa (grant 056708/Z/99).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This work was performed as part of an Interuniversity Attraction Pole Program.