Comment on Renshaw et al, page 3976
The generation of transgenic zebrafish with fluorescent neutrophils provides a new tool to study the dynamic contribution of granulocytes to acute inflammation and its pathological consequences.
Inflammation can only be studied in toto using in vivo models, primarily because of the diverse and dynamic cellular processes involved. Vascular integrity is secured, endothelial cells are activated, neutrophils (initially) and macrophages (later) egress from the circulation and localize to the inflammatory focus, and the adaptive immune system responds to foreign agents. Once the whole act is initiated, it must then resolve appropriately, or else pathological tissue damage will occur. While the individual molecular and cellular components of acute inflammation have been studied (eg, cytokine and chemokine biology, endothelial activation, maintaining the supply of phagocytes and their activation, chemotaxis), there is still much to be learned about the integrated process.FIG1,FIG2
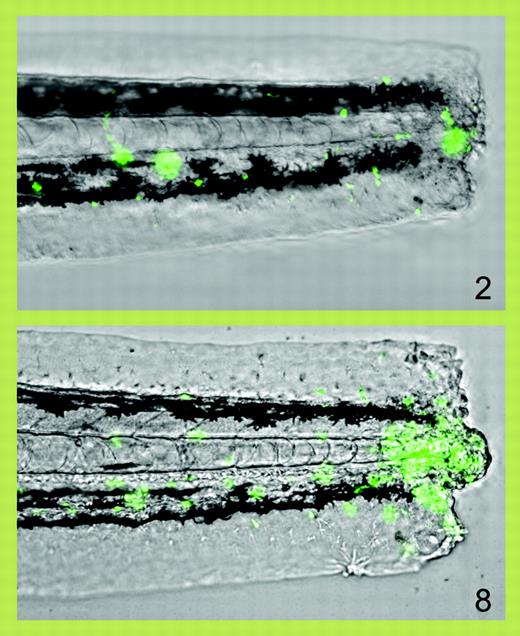
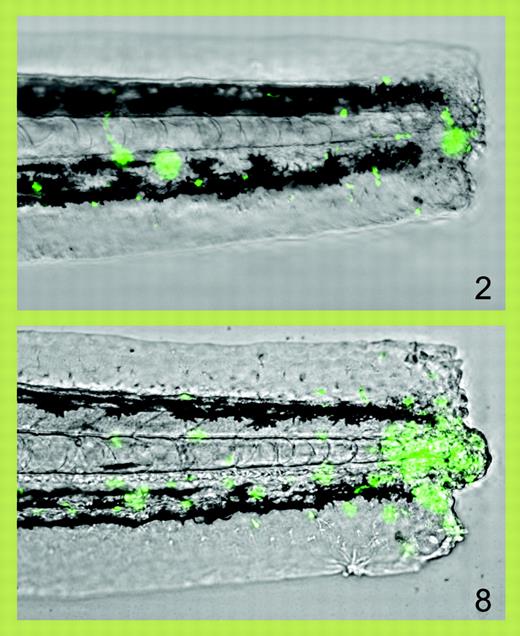
Progressive accumulation of fluorescent neutrophils (green) at 2 and 8 hours after transection of the tail of a zebrafish larva. See the complete figure in the article beginning on page 3976.
Progressive accumulation of fluorescent neutrophils (green) at 2 and 8 hours after transection of the tail of a zebrafish larva. See the complete figure in the article beginning on page 3976.
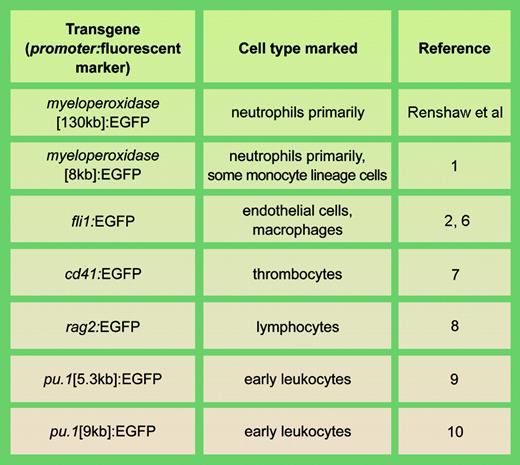
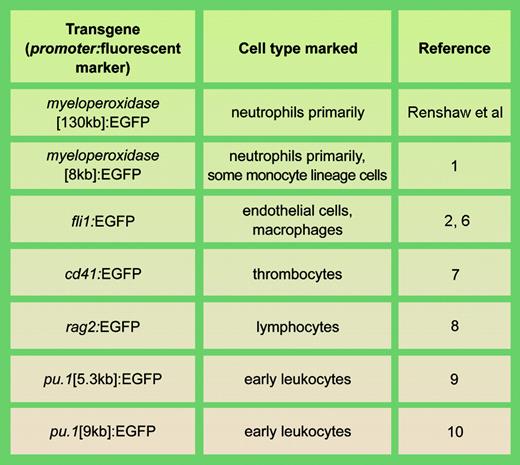
Examples of stable transgenic zebrafish lines with fluorescent cell types involved in actue inflammation. llustration by A. Y. Chen.
Examples of stable transgenic zebrafish lines with fluorescent cell types involved in actue inflammation. llustration by A. Y. Chen.
Animal models are required for this. In this issue of Blood, Renshaw and colleagues report the generation of a transgenic zebrafish expressing enhanced green fluorescence protein (EGFP) from the zebrafish myeloperoxidase promoter, resulting in fluorescent neutrophils. The optical transparency of whole zebrafish embryos means that fluorescent neutrophils can be followed in real time in vivo, and Renshaw and colleagues elegantly demonstrate how acute inflammation following a standardized experimental trauma (tail transection) can be followed in real time and quantitated, and the effects of pharmacological manipulation assessed (see figure). Renshaw et al used BAC recombination to construct a transgene placing EGFP under control of 130 kb of upstream regulatory sequence; very recently, another group has also reported the construction of a myeloperoxidase-promoter:EGFP-transgenic zebrafish, but using 8 kb of regulatory sequence.1 While both lines are very similar, that of Mathias et al1 shows early expression elsewhere in the tail, which may indicate that all the myeloperoxidase regulatory elements that restrict expression to leukocytes are not present in the smaller promoter or, alternately, may reflect a transgene integration site effect. Collectively, these 2 reports have already enriched our knowledge of the dynamics of acute inflammation, presenting new evidence that 2 mechanisms contribute to its resolution: neutrophil apoptosis (Renshaw et al) and their active departure from the inflammatory site.1
These new transgenic zebrafish are a welcome addition to the now impressive range of zebrafish with fluorescently marked cell types relevant to inflammation (see table). The involvement of fluorescent embryonic macrophages in acute inflammation has been studied in zebrafish with a fli1-driven EGFP,2 and the same line has been used to study endothelial regeneration after wounding.3 Although the collective data indicate that modeling inflammation in zebrafish is relevant to human inflammatory disease, several details require further exploration. There is some evidence that myeloperoxidase may not be a totally specific marker for zebrafish neutrophils, but may mark some early macrophage-lineage cells as well.1 At an ultrastructural level, zebrafish myeloperoxidase-containing neutrophil primary granules have a markedly different morphology from those of mammalian neutrophils4 ; whether this is reflected in a functional difference remains to be determined. It has also not been formally demonstrated whether zebrafish neutrophils are phagocytic.
Transgenic mice with EGFP-marked leukocytes driven from the lysozyme promoter are available5 and have been used to study leukocyte distribution in vivo. However, zebrafish offer unique opportunities to investigate the regulation of these complex cellular processes genetically by exploiting these fluorescently-marked lines in efficient, affordable forward genetic screens, and for identifying new therapeutic lead compounds modifying cellular contributions to acute inflammation by high-throughput chemical screening. With the spotlight now on inflammation in zebrafish, we can hope for new insights into its genetic regulation and, hopefully, the identification of novel anti-inflammatory therapeutic agents.
The author declares no competing financial interests. ▪