Abstract
Smad5 is known to transduce intracellular signals from bone morphogenetic proteins (BMPs), which belong to the transforming growth factor-β (TGF-β) superfamily and are involved in the regulation of hematopoiesis. Recent findings suggest that BMP4 stimulates proliferation of human primitive hematopoietic progenitors in vitro, while early progenitors from mice deficient in Smad5 display increased self-renewal capacity in murine embryonic hematopoiesis. Here, we evaluate the role of Smad5 in the regulation of hematopoietic stem cell (HSC) fate decisions in adult mice by using an inducible MxCre-mediated conditional knockout model. Surprisingly, analysis of induced animals revealed unperturbed cell numbers and lineage distribution in peripheral blood (PB), bone marrow (BM), and the spleen. Furthermore, phenotypic characterization of the stem cell compartment revealed normal numbers of primitive lin–Sca-1+c-Kit+ (LSK) cells in Smad5–/– BM. When transplanted in a competitive fashion into lethally irradiated primary and secondary recipients, Smad5-deficient BM cells competed normally with wild-type (wt) cells, were able to provide long-term reconstitution for the hosts, and displayed normal lineage distribution. Taken together, Smad5-deficient HSCs from adult mice show unaltered differentiation, proliferation, and repopulating capacity. Therefore, in contrast to its role in embryonic hematopoiesis, Smad5 is dispensable for hematopoiesis in the adult mouse.
Introduction
Transforming growth factor-β (TGF-β) superfamily members, including the TGF-βs, activins, and bone morphogenetic proteins (BMPs), regulate a wide variety of biological functions such as cell proliferation, differentiation, migration, and apoptosis in a context-dependent manner.1 Signaling by these growth factors is initiated when binding of the ligand induces the assembly of a heteromeric complex of type I and type II serine/threonine kinase receptors. The type I receptors, also known as activin receptor–like kinases (ALKs), are recruited by the type II receptors and activate specific receptor-regulated Smads (R-Smads; Smad1-Smad3, Smad5, and Smad8) by phosphorylation.2,3 Activated R-Smads then form complexes with the common partner Smad4 and translocate into the nucleus, where they participate in transcriptional regulation of target genes.2,3 Conversely, inhibitory Smads (I-Smads; Smad6 and Smad7) negatively regulate TGF-β signaling by competing with the R-Smads for receptor or Smad4 interaction, and by marking the receptors for degradation by recruiting the E3-ubiquitin ligase.2,4 Generally, Smad2 and Smad3 act downstream of the TGF-β and activin receptors, while Smad1, Smad5, and Smad8 primarily mediate BMP signals. In endothelial cells, however, TGF-β signals via both ALK5 and ALK1, inducing phosphorylation of Smad2/3 and Smad1/Smad5, respectively, playing an important role in balancing processes of angiogenesis.5,6 The relevance of TGF-β signaling mediated by Smad1/Smad5 in hematopoiesis is unclear, although Smad5 has been implicated in transducing TGF-β inhibitory signals in hematopoietic progenitors derived from human bone marrow (BM) and murine yolk sac.7,8
Several members of the TGF-β superfamily and the downstream Smads are involved in the regulation of fate decisions of hematopoietic progenitors and stem cells.9,10 Homozygous disruption of the Smad5 gene results in lethality at embryonic day (E) 9.5 to E11.5, mainly due to defects in angiogenesis.11,12 Hence, the role of BMP signaling in murine hematopoiesis has solely been studied in embryos, where Smad5 was shown to negatively regulate the proliferation and self-renewal of early progenitors during embryonic hematopoiesis.8 In humans, effects of BMP signaling in the regulation of hematopoietic stem cells (HSCs) have also been reported in early ontogeny. BMP4, in particular, has been shown to regulate HSC specification during development,13,14 and high concentrations of BMP4 maintain the proliferation of human cord blood (CB) HSCs in vitro.15 A recent study examining adult murine hematopoiesis has demonstrated an indirect effect of BMP on the number of HSCs.16 By knocking out the BMP receptor IA (ALK3), Zhang et al showed that impaired BMP signaling increases the niche size, and thereby enhances the number of HSCs.16
Here, for the first time, we have examined the effects of Smad5 deficiency in murine adult hematopoiesis. To circumvent lethality during embryonic development, and to study the role of Smad5 for HSC function and adult hematopoiesis, we have used a Cremediated conditional knockout model.17 Transgenic mice expressing Cre-recombinase under control of the interferon-inducible promoter Mx1 (MxCre)18 efficiently recombine floxed genes in the hematopoietic system.19 Our study reveals that Smad5-deficient animals have unperturbed cell numbers and lineage distribution in peripheral blood (PB), BM, and the spleen, as well as normal numbers of primitive lin–Sca-1+c-Kit+ (LSK) cells and committed progenitors in the BM. Furthermore, adult murine HSCs lacking Smad5 demonstrate normal self-renewal and differentiation capacity following bone marrow transplantation, indicating that Smad5 is dispensable for hematopoiesis in the adult mouse.
Materials and methods
Mice
Mice with a “floxed” Smad5 allele (loxP-flanked exon 2) for Cre-mediated conditional knockout have been previously described.17 Floxed mice were backcrossed 6 generations onto C57Bl6. Homozygous Smad5fl/fl mice were crossed with hemizygous MxCre;Smad5fl/+ mice to generate MxCre; Smad5fl/fl mice. Cre expression was induced with 3 intraperitoneal injections (at 2-day intervals) of 250 μg polyinositolic polycytidylic acid (pIC; Sigma-Aldrich, St Louis, MO). Genotyping for detection of wild-type (wt), floxed, and excised alleles was done using a 3-primer polymerase chain reaction (PCR) as described previously.17 The presence of MxCre was verified by PCR using the following primers: Cre forward, 5′-ACGAGTGATGAGGTTCGCAA-3′; and Cre reverse, 5′-AGCGTTTTCGTTCTGCCAAT-3′. Smad5fl/+ and Smad5fl/fl mice lacking MxCre still had 2 intact alleles of Smad5 after the pIC induction, and were hence used as wt controls. Mice were housed and bred in ventilated racks in a barrier facility. All animal experiments were approved by Lund University's Animal Ethics Committee.
Western blot analysis
BM cells from induced MxCre;Smad5fl/fl and wt mice were sorted for the pan-hematopoietic marker CD45. Whole-cell extracts were boiled for 10 minutes in 2 × sample loading buffer (30 μL/L × 106 cells) before they were separated on 8% SDS-acrylamide gels and transferred to PVDF membranes (Amersham Biosciences, Buckinghamshire, United Kingdom). Membranes were blocked in phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBS-T) containing 5% bovine serum albumin (BSA; Rockland Immunochemicals, Gilbertsville, PA) before incubation with antibodies (Smad5, D20, from Santa Cruz Biotechnology, Santa Cruz, CA; Smad1, from Cell Signaling Technology, Danvers, MA; and actin, from BD Bioscience Pharmingen, Franklin Lakes, NJ). The binding of primary antibody was detected by horseradish peroxidase–conjugated secondary antibodies (antimouse and antirabbit, from Amersham Biosciences; and antigoat, from Santa Cruz Biotechnology), followed by a chemiluminescence Western blot kit (PerkinElmer Life Sciences, Wellesley, MA).
Cell preparations
PB was collected from the tail vein and analyzed on a blood cell analyzer (Boule Medonice CA 530-16, Kobe, Japan) to determine cell counts. BM and spleen were crushed and passaged through a 70-mm cell strainer (Becton Dickinson [BD], Bedford, MA) to obtain single-cell suspensions. Cells were kept in PBS (Gibco-BRL, Paisley, United Kingdom) containing 2% fetal calf serum (FCS; Gibco-BRL). When necessary, red blood cells were lysed with ammonium chloride (NH4Cl; Stem Cell Technologies, Vancouver, BC, Canada). For lineage depletion, cells were incubated with unconjugated rat antibodies against murine CD4, CD8, CD5, Gr1, Mac1, B220, and TER119 (BD Bioscience Pharmingen). Lin+ cells were removed with a magnetic particle concentrator (MPC-6; Dynal Biotech, Oslo, Norway) following incubation with sheep anti–rat immunoglobulin G (IgG) crystallizable fragment (Fc)–conjugated immunomagnetic beads (Dynal Biotech).
Flow cytometry
Rat antibodies against murine Mac1, Gr1, B220, CD3, Sca-1, c-kit, CD34, CD45.1 (Ly5.1), and CD45.2 (Ly5.2), either unconjugated or conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), or biotin, were obtained from BD Biosciences Pharmingen. Unconjugated antibodies were detected with tricolor conjugated goat F(ab′)2 anti–rat IgG (H+L) (Caltag Lab, Burlingame, CA), while biotinylated antibodies were detected with streptavidin-APC (BD Biosciences Pharmingen). Dead cells were excluded through staining with 7–aminoactinomycin D (7-AAD; Sigma-Aldrich). Cells were sorted by fluorescence-activated cell sorting (FACS) on a FACS Vantage Cell Sorter (BD) or analyzed on a FACS Calibur (BD). Results were analyzed with CellQuest software (BD) or FlowJo software (Tree Star, Ashland, OR).
Hematopoietic progenitor assays
For granulocyte-macrophage colony-forming units (CFU-GMs) 30 000 fresh BM cells were plated per milliliter of methylcellulose medium (M3231; Stem Cell Technologies) containing 50 ng/mL murine stem cell factor (SCF; Amgen, Thousand Oaks, CA), 10 ng/mL murine interleukin 3 (IL-3; Peprotech, Rocky Hill, NJ), and 10 ng/mL human IL-6 (gift from Novartis, Basel, Switzerland) in 35-mm Petri dishes. For erythroid burst-forming units (BFU-Es), 150 000 BM cells were plated per milliliter of serum-free methylcellulose (M3236; Stem Cell Technologies) supplemented with 50 ng/mL SCF, 50 ng/mL human thrombopoietin (TPO; Peprotech), and 5 U/mL human erythropoietin (Epo; Janssen-Cilag AB, Sollentuna, Sweden). CFU-GMs were scored on day 7 and BFU-Es were scored on days 9 and 10. CFU-GMs were also plated with and without human TGF-β1 at indicated doses (Peprotech).
Single-cell cultures
LSK cells from induced mice were isolated by FACS (FACS Vantage Cell Sorter; BD) and plated in 96-well plates at 1 cell/well. Cells were grown in 50 μL serum-free medium (X-vivo 15; BioWhittaker, Walkersville, MD) supplemented with 1% BSA (Stem Cell Technologies), 100 IU/mL penicillin, 100 mg/mL streptomycin (Gibco-BRL), 2 mM l-glutamine (Gibco-BRL), and 10–4 M 2-mercaptoethanol (Sigma-Aldrich). Cytokines used were: SCF (25 ng/mL; Amgen), TPO (25 ng/mL; Peprotech), human Flt-3 ligand (FL; 25 ng/mL; R&D Systems, Abingdon, United Kingdom), and human TGF-β1 (Peprotech). Fresh supplemented medium containing cytokines (50 μL) was added at day 6 and proliferating cell clones were scored after 12 days.
Competitive transplantations
BM cells (5 × 105) from pIC-induced MxCre;Smad5fl/fl and wt mice (Ly5.2) were transplanted together with 5 × 105 Bl6/SJL competitor BM cells (Ly5.1) into lethally irradiated (900 cGy) Bl6/SJL recipient mice (3 recipients/donor). After 16 weeks, recipients were killed and half of a femur-equivalent of BM was transplanted into lethally irradiated secondary recipients. PB was collected at several time points from mice receiving transplants to determine the Ly5.1 and Ly5.2 reconstitution levels and lineage distribution by FACS analysis.
Limiting dilution transplantations
Limited doses (5000, 10 000, 25 000, and 50 000 cells) of unfractionated BM from pIC-induced wt and MxCre;Smad5fl/fl mice (Ly5.2; 1 donor and 2 donors, respectively) were mixed with 2 × 105 support BM cells from C57Bl6/SJL mice (Ly5.1/5.2) and transplanted into lethally irradiated (900 cGy) C57Bl6/SJL recipient mice (3-8 recipients). At 12 weeks PB was taken from recipient mice, and the donor contribution and lineage distribution was analyzed by FACS.
Statistical analysis
The significance of results was analyzed using the Student t test. A P value less than .05 was considered significant.
Results
Smad5 is efficiently deleted in induced MxCre Smad5 conditional knockout mice
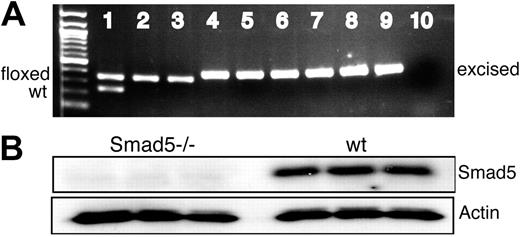
To confirm Cre/loxP recombination of exon2 in the Smad5 gene, MxCre;Smad5fl/fl and wt mice were injected intraperitoneally with pIC. Subsequently, BM cells were plated in methylcellulose, and after 7 days of culture individual colonies were isolated and PCR analysis was used to assess the efficiency of the deletion of Smad5 in BM progenitors. Consistent with previous reports testing MxCre-mediated deletion in hematopoietic colonies,19 exon 2 was deleted in essentially all colonies tested (> 98%; Figure 1A). To confirm Smad5 deletion at the protein level, BM cells from induced MxCre;Smad5fl/fl and wt mice were sorted for the pan-hematopoietic marker CD45 and analyzed by Western blot. As shown in Figure 1B, no Smad5 protein was detected in BM cells from induced MxCre;Smad5fl/fl mice, confirming the creation of homozygous null alleles after induction in hematopoietic cells. Since BMP signals can be transduced by Smad1, Smad5, and Smad8, we wanted to examine whether the expression levels of Smad1 and Smad8 were altered in the Smad5-deficient cells. Smad8 is not expressed in primitive hematopoietic cells,20 and was not up-regulated at the RNA level in Smad5 knockout cells. Similarly, the level of Smad1 expression remained unchanged both at RNA and protein levels (data not shown).
Smad5-deficient hematopoietic progenitors retain normal proliferation and differentiation capacity in vitro
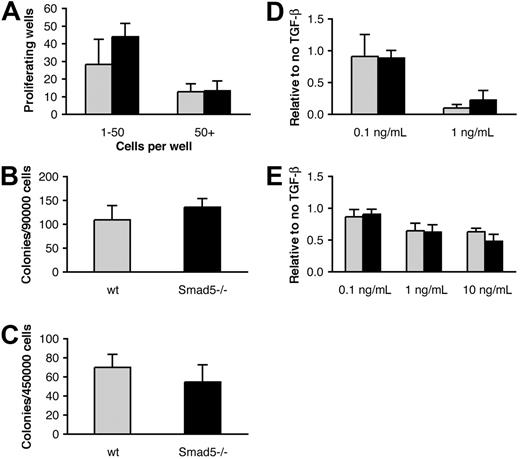
Smad5 has been suggested to negatively regulate the proliferation of early multipotent hematopoietic progenitors derived from yolk sac and embryonic bodies in vitro.8 To investigate the effect of Smad5 deficiency on the proliferation capacity of adult hematopoietic progenitors, we performed single-cell culture assays. Primitive LSK BM cells were isolated by FACS and plated in 96-well plates at 1 cell/well. The cells were grown in serum-free medium supplemented with SCF, TPO, and FL, and proliferating wells were scored after 12 days. As shown by Figure 2A, there was no significant difference between Smad5-deficient and wt progenitors in the number of wells containing either low-proliferating cells (1-50 cells/well) or high-proliferating cells (> 50 cells/well). In addition, we analyzed the ability of Smad5-deficient BM to form committed progenitors by plating BM cells in cytokine-containing semisolid medium. These experiments revealed no difference in size or number of myeloid (CFU-GM) or erythroid (BFU-E) colonies formed (Figure 2B-C), suggesting that neither the number of hematopoietic progenitors nor their capacity to proliferate or differentiate in vitro is affected by the loss of Smad5.
Smad5-deficient progenitors show normal sensitivity to TGF-β1 inhibition
Recent findings have shown that BMP4 stimulates proliferation and colony formation of primitive human CB progenitors in vitro.15 While murine BM cells propagate a BMP4 signal through phosphorylation of Smad1/Smad5, BMP4 does not have analogous effects on murine progenitor proliferation or colony formation.20 However, Smad5 has also been reported to transduce inhibitory signals from TGF-β1 in hematopoietic progenitors from both human BM7 and murine embryonic bodies.8 Since TGF-β1 has a well-documented inhibitory effect on murine progenitors in vitro, we wanted to examine the effect of Smad5 deficiency in response to TGF-β1 on hematopoietic progenitors from adult mice. We therefore purified LSK cells by FACS and seeded them as single cells in 96-well plates in serum-free medium containing SCF, TPO, and FL, with or without TGF-β1 (0.1 or 1 ng/mL). However, no significant difference was observed between Smad5-deficient and wt progenitors in numbers of wells with proliferating cells, number of cells per well (Figure 2A; data not shown), or in their response to TGF-β1 inhibition at any of the concentrations used (Figure 2D). In addition, we plated whole BM for CFU-GM formation with and without 3 different concentrations of TGF-β1 (0.1, 1, and 10 ng/mL). Again, the results showed no difference either in the number of colonies formed (Figure 2B; data not shown) or in their sensitivity to TGF-β1 (Figure 2E). Taken together, this suggests that Smad5 is not required for TGF-β1 inhibition of hematopoietic progenitors from adult mice in vitro.
Smad5 is efficiently deleted inMxCre;Smad5fl/flconditional knockout mice. (A) Representative figure of a PCR screening of individual hematopoietic colonies from induced and uninduced MxCre;Smad5fl/fl and wt mice. The primer pairs 1 and 2 and 1 and 3 were used to detect wt (235 bp), floxed (349 bp), and excised (387 bp) alleles, respectively, as previously described.17 Lane 1 shows DNA from an uninduced Smad5fl/+ mouse lacking Cre (identical bands when induced; not shown); lanes 2 and 3, DNA from 2 different uninduced MxCre;Smad5fl/fl mice; lanes 4 through 9, colony DNA from the same MxCre;Smad5fl/fl mice after induction of Cre recombination (3 representative colonies/mouse); and lane 10, negative control. Exon 2 was deleted in more than 98% of all colonies tested (n = 446). (B) Western blot analysis of Smad5 protein in CD45+ BM cells from 3 different induced MxCre;Smad5fl/fl and wt mice, demonstrating the lack of Smad5 protein in Cre-recombined cells. Actin was used as loading control.
Smad5 is efficiently deleted inMxCre;Smad5fl/flconditional knockout mice. (A) Representative figure of a PCR screening of individual hematopoietic colonies from induced and uninduced MxCre;Smad5fl/fl and wt mice. The primer pairs 1 and 2 and 1 and 3 were used to detect wt (235 bp), floxed (349 bp), and excised (387 bp) alleles, respectively, as previously described.17 Lane 1 shows DNA from an uninduced Smad5fl/+ mouse lacking Cre (identical bands when induced; not shown); lanes 2 and 3, DNA from 2 different uninduced MxCre;Smad5fl/fl mice; lanes 4 through 9, colony DNA from the same MxCre;Smad5fl/fl mice after induction of Cre recombination (3 representative colonies/mouse); and lane 10, negative control. Exon 2 was deleted in more than 98% of all colonies tested (n = 446). (B) Western blot analysis of Smad5 protein in CD45+ BM cells from 3 different induced MxCre;Smad5fl/fl and wt mice, demonstrating the lack of Smad5 protein in Cre-recombined cells. Actin was used as loading control.
Smad5-deficient progenitors possess unperturbed differentiation and proliferation and show normal sensitivity to TGF-β1 inhibition in vitro. (A) Single-cell culture evaluating the proliferation capacity of hematopoietic progenitors. Primitive LSK cells from induced mice were purified by FACS and cultured in supplemented serum-free medium containing SCF, TPO, and FL for 12 days (n = 4). Colony assays were performed to evaluate the ability of committed progenitors to form (B) myeloid and (C) erythroid colonies (n = 3). (D-E) Proliferation and colony assay examining the sensitivity of primitive hematopoietic cells and myeloid progenitors to TGF-β1 inhibition, respectively. (D) LSK cells were grown as described for panel A with 0, 0.1, or 1 ng/mL TGF-β1 (n = 3). (E) CFU-GMs were plated with 0, 0.1, 1, or 10 ng/mL TGF-β1 (n = 12, 5, 8, and 4 for the different concentrations, respectively). Data represent mean values ± SD. ▦ indicates wt cells; ▪, Smad5-deficient cells.
Smad5-deficient progenitors possess unperturbed differentiation and proliferation and show normal sensitivity to TGF-β1 inhibition in vitro. (A) Single-cell culture evaluating the proliferation capacity of hematopoietic progenitors. Primitive LSK cells from induced mice were purified by FACS and cultured in supplemented serum-free medium containing SCF, TPO, and FL for 12 days (n = 4). Colony assays were performed to evaluate the ability of committed progenitors to form (B) myeloid and (C) erythroid colonies (n = 3). (D-E) Proliferation and colony assay examining the sensitivity of primitive hematopoietic cells and myeloid progenitors to TGF-β1 inhibition, respectively. (D) LSK cells were grown as described for panel A with 0, 0.1, or 1 ng/mL TGF-β1 (n = 3). (E) CFU-GMs were plated with 0, 0.1, 1, or 10 ng/mL TGF-β1 (n = 12, 5, 8, and 4 for the different concentrations, respectively). Data represent mean values ± SD. ▦ indicates wt cells; ▪, Smad5-deficient cells.
Steady-state hematopoiesis is unperturbed in induced MxCre;Smad5fl/fl
To study the long-term effect of Smad5 deficiency during steady-state hematopoiesis, MxCre;Smad5fl/fl and wt mice were induced at 6 to 12 weeks of age, after which they were monitored daily and weighed every 2 weeks. Weight gain of Smad5 knockout mice was similar to that of wt mice (data not shown), and the mice remained healthy for up to 16 weeks after induction, when experiments were terminated. PB analysis of red blood cell (RBC) and white blood cell (WBC) counts revealed values within normal ranges that were not different from those of wt control animals, suggesting that Smad5 deletion had no effect on PB homeostasis (Table 1). Furthermore, FACS analysis of PB, BM, and the spleen showed unperturbed ratios of myeloid cells (Mac-1, Gr-1), B-cells (B220), and T cells (CD3) (Figure 3A-C). In addition, analysis of BM showed normal numbers of both total cells and the primitive LSK CD34– cell population that is highly enriched for HSCs (Table 1). Taken together, these results suggest that Smad5 is dispensable for the maintenance of the hematopoietic system under steady-state conditions.
Normal competitive repopulation ability and lineage distribution in vivo after BM transplantation of Smad5-deficient cells
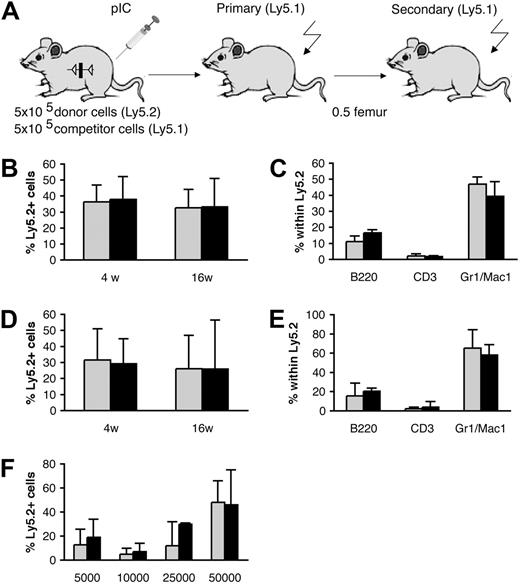
Since Smad5 has been implied to negatively regulate the proliferation and self-renewal of multipotent progenitors during murine embryonic hematopoiesis,8 we asked if Smad5-deficient HSCs from adult mice exhibited an increased regenerative ability in vivo. To study this, 5 × 105 BM cells from induced MxCre;Smad5fl/fl and wt mice (Ly5.2) were transplanted into lethally irradiated recipients (Ly5.1) together with 5 × 105 competitor cells (Ly5.1; Figure 4A). The recipients were followed for 16 weeks and PB was collected at several time points. FACS analysis of the Ly5.1/Ly5.2 contributions in PB and BM showed no significant difference in the competitive repopulation ability in either short-term (PB, 4 weeks: Smad5–/–, 37.9% ± 14.3% Ly5.2; and wt, 36.4% ± 10.5% Ly5.2) or long-term (BM, 16 weeks: Smad5–/–, 33.2% ± 17.7% Ly5.2; and wt, 32.6% ± 11.5% Ly5.2; n = 5) reconstitution of the primary recipients (Figure 4B). In agreement with the unaltered repopulative capacity of Smad5–/– BM, the number of primitive LSK cells within the donor-derived BM population remained unchanged (Table 2). Furthermore, the contribution of donor-derived cells to the myeloid and lymphoid compartments was unaltered in both PB (data not shown) and BM (Figure 4C). To further investigate the effect of Smad5 deficiency on the self-renewal capacity of HSCs, half of a femur-equivalent of BM from primary recipients was transplanted into lethally irradiated secondary recipients. Subjecting the HSCs to this additional stress did not result in any difference in the repopulative capacity of Smad5–/– HSCs compared with wt (PB, 4 weeks: Smad5–/–, 29.2% ± 15.7% Ly5.2; and wt, 31.5% ± 19.5% Ly5.2; BM, 16 weeks: Smad5–/–, 25.9% ± 30.6% Ly5.2; and wt, 28.1% ± 18.4% Ly5.2; n = 5) (Figure 4D). Again, the donor-derived cells from both induced wt and MxCre;Smad5fl/fl mice showed similar numbers of LSK cells (Table 2), as well as normal contribution to both myeloid and lymphoid lineages in PB (data not shown) and BM (Figure 4E) as determined by FACS.
Normal hematopoietic lineage distribution in inducedMxCre; Smad5fl/flmice. FACS analysis on cells derived from PB (A), BM (B), and the spleen (C) taken 16 weeks after induction using markers for myeloid cells (Mac1, Gr1), B cells (B220), and T cells (CD3) (n = 3). Data represent mean values ± SD. ▦ indicates wt cells; ▪, Smad5-deficient cells.
Normal hematopoietic lineage distribution in inducedMxCre; Smad5fl/flmice. FACS analysis on cells derived from PB (A), BM (B), and the spleen (C) taken 16 weeks after induction using markers for myeloid cells (Mac1, Gr1), B cells (B220), and T cells (CD3) (n = 3). Data represent mean values ± SD. ▦ indicates wt cells; ▪, Smad5-deficient cells.
Normal competitive repopulation ability and lineage distribution in vivo after BM transplantation ofSmad5–/–cells. (A) Schematic figure showing the experimental layout of the primary and secondary transplantations. Competitive repopulation ability was measured as Ly5.2/Ly5.1 contribution. Shown is the short-term reconstitution in PB at 4 weeks, and long-term reconstitution at 16 weeks in BM from primary (B) and secondary (D) recipients. The distribution of myeloid cells (Mac1, Gr1), B cells (B220), and T cells (CD3) within the Ly5.2 population in BM from primary (C) and secondary (E) recipients was analyzed by FACS. Data represent mean values ± SD (n = 5 donors, 15 recipients). (F) Limiting dilution transplantation revealing similar repopulative capacities between BM cells from induced wt and MxCre;Smad5fl/fl mice in all doses analyzed (5000, 10 000, 25 000, and 50 000 donor cells). Data represent mean values ± SD; n = 3 to 4 recipients per dose for wt and 7 to 8 recipients per dose for Smad5-deficient cells. ▦ indicates wt cells; ▪, Smad5-deficient cells.
Normal competitive repopulation ability and lineage distribution in vivo after BM transplantation ofSmad5–/–cells. (A) Schematic figure showing the experimental layout of the primary and secondary transplantations. Competitive repopulation ability was measured as Ly5.2/Ly5.1 contribution. Shown is the short-term reconstitution in PB at 4 weeks, and long-term reconstitution at 16 weeks in BM from primary (B) and secondary (D) recipients. The distribution of myeloid cells (Mac1, Gr1), B cells (B220), and T cells (CD3) within the Ly5.2 population in BM from primary (C) and secondary (E) recipients was analyzed by FACS. Data represent mean values ± SD (n = 5 donors, 15 recipients). (F) Limiting dilution transplantation revealing similar repopulative capacities between BM cells from induced wt and MxCre;Smad5fl/fl mice in all doses analyzed (5000, 10 000, 25 000, and 50 000 donor cells). Data represent mean values ± SD; n = 3 to 4 recipients per dose for wt and 7 to 8 recipients per dose for Smad5-deficient cells. ▦ indicates wt cells; ▪, Smad5-deficient cells.
In addition, we performed a limiting dilution transplantation using 4 different doses, ranging from 5000 to 50 000 cells, of unfractionated BM from induced MxCre;Smad5fl/fl and wt mice (Ly5.2). These cells were mixed with 2 × 105 support BM cells from C57Bl6/SJL mice (Ly5.1/5.2) and transplanted into lethally irradiated C57Bl6/SJL recipient mice. PB was collected from recipient mice at 12 weeks after transplantation, and the donor contribution was analyzed by FACS. None of the doses resulted in any significant difference in Ly5.2 contribution between recipients receiving wt or Smad5-deficient BM (Figure 4F), which further suggests that Smad5–/– and wt HSCs have equal potential to repopulate irradiated recipients. Conclusively, these results suggest that Smad5 is dispensable for differentiation, self-renewal, and short- and long-term hematopoietic repopulation capacity of HSCs in adult mice.
Discussion
Because the conventional Smad5 knockout mouse dies during embryonic development, all previous knowledge about the role of Smad5 in murine hematopoiesis has been inferred from studies on cells of embryonic origin. Smad5 negatively regulates the proliferation of early multipotent hematopoietic progenitors derived from yolk sac and embryonic bodies in vitro.8 In the same study, Smad5-deficient embryonic bodies gave rise to decreased numbers of erythroid progenitors, while myeloid progenitors were unaffected.8 Another murine study, however, showed a 2-fold increase in the capacity of Smad5–/– yolk sac cells to form CFU-GMs, while their capacity to form erythroid colonies was unchanged.12 Although the effect of Smad5 deficiency on differentiation in embryonic hematopoiesis is not conclusive, Smad5 seems to play a role in embryonic hematopoietic development. In contrast, our study clearly shows that Smad5 is dispensable in adult hematopoiesis. We demonstrate normal self-renewal and repopulative capacity of Smad5-deficient HSCs after BM transplantation, thus indicating that Smad5 is not needed for hematopoietic reconstitution in adult mice. In addition, hematopoietic progenitors showed unaltered proliferation ability in both in vitro cultures and steady-state hematopoiesis. Furthermore, we observed normal differentiation of Smad5-deficient hematopoietic progenitors under steady-state conditions both in vitro and in vivo, and the unaltered potential of HSCs to reconstitute all lineages in lethally irradiated recipients after BM transplantation.
BMP signaling, which is mediated by Smad1, Smad5, and Smad8, has been demonstrated to constrain HSC numbers indirectly by negatively regulating the niche size.16 The same study demonstrated normal reconstitution ability of ALK3-deficient cells in wt recipients, although this did not adequately address the loss of BMP signals in HSCs intrinsically, since ALK3 is not expressed by these cells.16,20 In our steady-state experiments, the HSCs reside in a Smad5-deficient niche as MxCre-induced deletion is efficient in the osteoblastic cells in trabecular bone. Although this is the same Cre strategy used by Zhang et al,16 we did not observe an increase in numbers of primitive hematopoietic LSK cells. One possible explanation for this could be that BMP regulation of the niche space may be critically relevant to early stages of bone development. Zhang et al disrupted BMP signaling at a younger age, 3 or 21 days, compared with induction at 6 weeks of age in our study. Alternatively, related Smads may functionally compensate for the lack of Smad5 in our conditional knockout mice. Functional compensation can explain why mutants lacking BMP2, BMP4, or the upstream ALK3 receptor display earlier and/or more severe phenotypes than the Smad1 or the Smad5 knockouts.21-23 Even though Smad1 and Smad5 possess inherent specificities, shown by different expression and distribution patterns,24-26 as well as different knockout phenotypes,11,12,25,27-29 it is quite probable that the absence of effects in our study might be due to redundancy with Smad1. It was shown recently that Smad1+/–;Smad5+/– mutants, in contrast with Smad1 or Smad5 single heterozygotes, die and display defects that closely resemble those seen in Smad1 or Smad5 homozygous mutants.30 Thus, Smad1 and Smad5 share equivalent functional activities in the early embryo and can work cooperatively.
Interestingly, the phenotype of the Smad5–/– yolk sac resembles the phenotype in TGF-β131 and TGF-β receptor I (TRβI)32 and TRβII knockouts,33 suggesting that Smad5 might mediate TGF-β signals during angiogenesis of the developing embryo.11,12 It has been reported that Smad5, but not Smad1 or Smad8, can bind to the Smad-binding element (SBE) at similar levels to that of the TGF-β–specific Smad3 and Smad4, further suggesting that Smad5 may play a role in TGF-β signaling.34 Accordingly, Smad5 has been reported to transduce inhibitory signals from TGF-β1 in proliferation of hematopoietic progenitors from human CD34+ BM,7 and primitive multipotent progenitors derived from murine embryonic bodies.8 However, in both studies loss of Smad5 was unable to reverse inhibition caused by higher doses of TGF-β1, suggesting a predominating role for other Smads or pathways in the transmission of its inhibitory effects at high concentrations. Our study reveals unaltered TGF-β sensitivity of both proliferation and colony formation of hematopoietic progenitors lacking Smad5, demonstrating that Smad5 is not necessary for TGF-β1 inhibition of adult murine hematopoietic progenitors in vitro.
Our results suggest that the developmental context is an important factor in defining the role for Smad5 in hematopoiesis. In humans, a role for BMP signaling in hematopoiesis has also mainly been reported early in ontogeny. Apart from being essential for regulation of early hematopoietic development,13 BMP4 has been shown to promote hematopoietic differentiation of human embryonic stem cells14 and to stimulate proliferation and colony formation of human primitive progenitors purified from CB in vitro.15 However, BMP4 does not have a stimulatory effect on either proliferation or colony formation of hematopoietic progenitors from murine BM.20 This discrepancy in response to BMP4 may depend on biological differences between mouse and human, but also on ontogenic differences. In fact, Bhatia et al15 demonstrated significant expression of ALK-3 and ALK-6 in HSCs derived from human CB, while the expression level of these BMP type I receptors in BM HSCs was very low to undetectable. The absence of these receptors has also been documented in mouse BM.20 Together this implies that cells of early ontogeny may reflect a unique population of HSCs with a distinct response to growth factors.
To summarize, we have for the first time evaluated the effects of the deletion of Smad5 in adult murine hematopoiesis, and have found that Smad5 is dispensable for normal hematopoiesis in the adult mouse both in vitro and in vivo. Because Smad1 may be able to compensate for the loss of Smad5, it will be important to analyze the distinct versus overlapping roles of Smad1 and Smad5 in adult hematopoiesis.
Authorship
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 8, 2006; DOI 10.1182/blood-2006-02-003384.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Professor Mikael Sigvardsson, Dr Jonas Larsson, and Dr Taiju Utsugisawa for helpful advice and discussions; the personnel in the animal facility for taking care of the mice; and Anna Fossumand Zhi Ma for expert assistance with cell sorting. L.U. and A.Z. are indebted to Prof Danny Huylebroeck for support. A.Z. was holder of a postdoctoral mandate of Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO-V).
This work was supported by the Swedish Cancer Society, the European Commission (INHERINET and CONCERT grants to develop gene therapy for HSCs), the Swedish Gene Therapy Program, the Swedish Medical Research Council, Crafoordska Stiftelsen, the Swedish Children Cancer Foundation, a Clinical Research Award from Lund University Hospital, and European Union (EU) grant T-Angiovasc QLG1-CT-2001-01032. The Joint Program on Stem Cell Research is supported by the Juvenile Diabetes Research Foundation and the Swedish Medical Research Council. The Lund Stem Cell Center is supported by a Center of Excellence grant in life sciences from the Swedish Foundation for Strategic Research.