Abstract
There are limited data on the effects of drug levels on therapeutic success in fungal infections. Low itraconazole trough levels have been associated with breakthrough fungal infections (
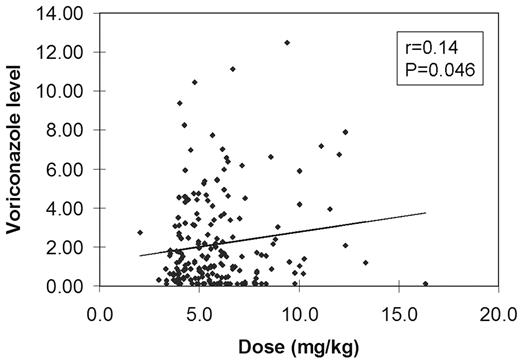
The table below shows the relationship between levels and dose. While the amount of drug administered in mg/kg was significantly higher when the levels were >5.0 μg/mL, there was no consistent relationship between dose and level below that threshold.
| Voriconazole level . | n . | Dose (mg) . | Dose (mg/kg) . | P (Dose in mg/kg; compared to the >5.0 level group) . |
|---|---|---|---|---|
| <0.2 (undetectable) | 30 (15%) | 400 (200–800) | 5.6 (3.5–11.5) | 0.008 |
| 0.2–0.5 | 25 (12%) | 400 (400–800) | 6.2 (3.8–10.2) | 0.18 |
| >0.5 to 2.0 | 70 (35%) | 400 (400–800) | 5.2 (3.7–13.3) | 0.062 |
| >2.0 to 5.0 | 53 (26%) | 400 (400–800) | 4.9 (2.0–9.8) | 0.0008 |
| >5.0 | 23 (11%) | 400 (400–800) | 6.9 (3.4–16.3) |
| Voriconazole level . | n . | Dose (mg) . | Dose (mg/kg) . | P (Dose in mg/kg; compared to the >5.0 level group) . |
|---|---|---|---|---|
| <0.2 (undetectable) | 30 (15%) | 400 (200–800) | 5.6 (3.5–11.5) | 0.008 |
| 0.2–0.5 | 25 (12%) | 400 (400–800) | 6.2 (3.8–10.2) | 0.18 |
| >0.5 to 2.0 | 70 (35%) | 400 (400–800) | 5.2 (3.7–13.3) | 0.062 |
| >2.0 to 5.0 | 53 (26%) | 400 (400–800) | 4.9 (2.0–9.8) | 0.0008 |
| >5.0 | 23 (11%) | 400 (400–800) | 6.9 (3.4–16.3) |
These data show that in adult patients getting standard doses of voriconazole orally, the drug levels are highly variable. Based on the data on the FDA files, 27% of these levels would be associated with a lower likelihood of response. Based on the data of Smith et al, 65% of these levels would be associated with a higher likelihood of failure. We suggest that future voriconazole studies should incorporate prospective therapeutic drug monitoring, and that pending further clarification, consideration should be given to checking levels in patients receiving the drug for confirmed, life-threatening fungal infections.
Disclosures: Voriconazole for prophylaxis of fungal infections.
Author notes
Corresponding author