Abstract
The current FACT/NetCord cord blood (CB) standards require testing of the final CB product for the presence of anaerobic and aerobic bacterial and fungal contamination. Previously, we have reported a reduced rate of microbial contamination when testing the final concentrated product with a single pediatric (0.8%) compared to the plasma waste fraction with both aerobic and anaerobic (5.1%) blood culture bottles (Cytotherapy 7:Suppl 1, Abstr 88, 2005). The issue of sensitivity of bottle type and small volume available from the final product were highlighted. Subsequently, a combination of both protocols was introduced as the routine screening procedure.
Aim: To analyse microbial contamination of CB donations using three blood culture bottles and two differing test sample specimens as the screening procedure.
Method: Between Jan 2005 and Jul 2006, 1,715 processed CB units were tested for the presence of microbial contamination:
after separation from the white cell rich product, 10 mL of resulting plasma waste fraction was aseptically inoculated into each of an adult anaerobic (An) and adult aerobic (Ae) FAN BacT/Alert blood culture bottle (bioMerieux) and
after completion of processing and prior to freezing, 1 mL sample of the final product was inoculated into a paediatric (Pd) FAN BacT/Alert blood culture bottle (bioMerieux).
The bottles were incubated as per the manufacturer’s instructions for a maximum of five days. Bottles indicating positive growth were subcultured and species identification performed.
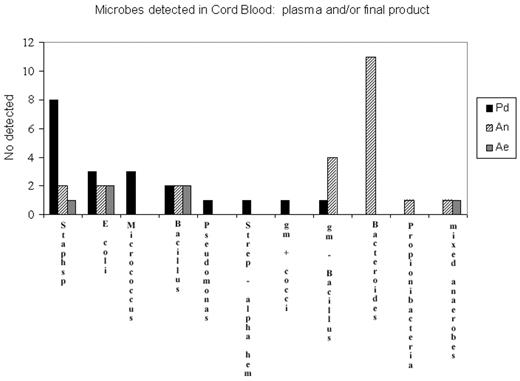
Results: A total of 46 CB units tested positive for bacterial growth, giving a microbial contamination rate of 2.68%. No fungal contamination was reported. Of these, 23 (50%) tested positive only in the plasma waste fraction, 18 (39%) tested positive only in the final product, and only 6.5% of CB units were positive in all three bottles. Of the 23 CB units positive from the plasma fraction, 20 had growth only in the anaerobic bottle and 2 had growth only in the aerobic bottle. Microbes were detected in the plasma fraction and not in the final product, and vice versa, and microbes detected in the plasma fraction were generally different to those detected in the final product. The plasma fraction detected mainly anaerobic organisms, the most common of which was Bacteroides (n=11). The Pd bottle grew aerobic and facultative anaerobes and missed the majority of the obligate anaerobes detected in the plasma fraction by either the Ae or An bottles. The most common organism detected in the Pd bottle was staphylococcus sp (n=8), of which only two were detected in the plasma waste fraction using either the anaerobic or aerobic bottles.
Microbes dectected in Cord Blood; plasma and/or final product
Conclusions: This data indicates that to optimise microbial detection in CB units, test protocols should include screening of both the plasma waste fraction and final product; and further, protocols should include testing with systems that promote growth of both anaerobic and aerobic organisms. It is not clear from this study whether microbe-positive CBs were not detected in the two test sources of sample (plasma vs final product) due to
inability of some organisms to grow in specific bottles and /or
low sample volume issues or
whether microbes were sequestered during processing into one specific fraction.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author