Abstract
The development of effective therapeutic strategies for T-lymphoid neoplasms has been difficult partly due to their rarity and paucity of clinical trials. These neoplasms are generally refractory to traditional chemotherapy regimens and novel therapeutic strategies are needed. Alemtuzumab and pentostatin have response rates of 50% to 65% when used individually to treat various T-cell leukemias and lymphomas. However, most responses are partial and of limited duration. Immunosuppression and risk of opportunistic infections are their principal overlapping toxicities. We have treated 17 patients with T-lymphoid malignancies (10 T-PLL, 1 ATL, 2 T-ALL, 2 γδ-T cell hepatosplenic lymphoma, 2 T-LGL) with a combination of alemtuzumab 30 mg IV, 3 times weekly for up to 3 months and pentostatin 4 mg/m2 weekly × 4 followed by alternate weekly administration for up to 6 months. Prophylactic antibiotics including valacyclovir, fluconazole, and trimethoprim/sulfamethoxazole (or equivalents) were administered during the treatment and for 2 months after its completion. The median age of patients was 57 years (range 22 – 79 years), median white blood count was 54.1 × 109/L (range 0.6 – 279.5 ×109/L), and median serum β2M was 4.0 mg/L (range, 1.7 – 10.8 mg/L). Four patients had splenomegaly (1 to 5 cm), and 5 lymphadenopathy. Eleven had prior therapy (median 2, range 1 to 6 regimens). Eleven patients have responded (8 CR, 3 PR) for an overall response rate of 65% (including 8 of 10 T-PLL, 1 of 1 ATL, 0 of 2 T-ALL, 1 of 2 γδ-T cell hepatosplenic lymphoma and 1 of 2 T-LGL). Monoclonal T-cell receptor chain gene rearrangements were detected by PCR in 16 patients and became negative in 5 of 7 evaluable patients in CR. Median response duration has not been reached (range 0 to 78 weeks). Three patients have proceeded to allogeneic stem cell transplant, 4 (1 with ATL, 2 with T-PLL, and 1 with T-LGL) have died from disease progression after a response, and 6 were refractory to therapy. Opportunistic infections included reactivation of CMV in 6 patients (no disease), reactivation of HSV in 1 patient, recurrence of pre-existing Serratia pneumonia in 1 patient and Aspergillus pneumonia in 2 patients. Unexplained, marked and sustained pancytopenia occurred in 2 patients, 1 with T-LGL and 1 with T-PLL. Other toxicities were mainly grade 1 and 2 and included nausea, fever, fatigue, infusion reactions, edema, and shortness of breath. The combination of alemtuzumab and pentostatin is feasible and effective in T-cell neoplasms. Although infections including CMV reactivation are a concern, they may be minimized with adequate prophylactic antibiotic therapy.
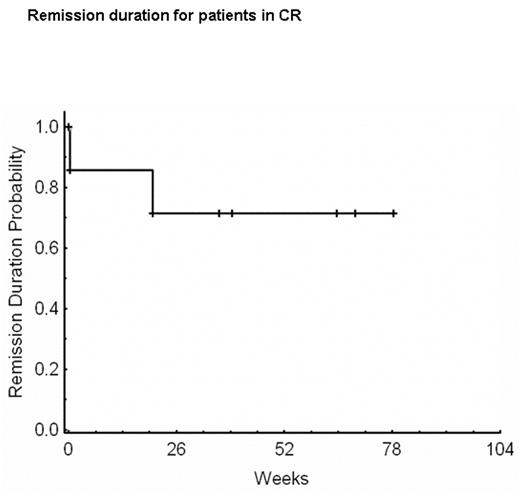
Remission duration for patients in CR
Disclosures: The presentation includes off-label use of pentostatin and alemtuzumab for the treatment of patients with T-lymphoid neoplasms.; Presenting author is in receipt of research funding from Berlex and Supergen pharmaceutical companies.
Author notes
Corresponding author