Abstract
Angioimmunoblastic T-cell Lymphoma (AITL) represents about 4–6% of all lymphomas, but up to 15–20% of all T-cell lymphomas. AITL usually presents in the 6th to 7th decade of life as an advanced stage, systemic disease with B-symptoms, lymphadenopathy, bone marrow involvement, rash, and autoimmune abnormalities. The disease follows an aggressive course and typically portends a poor prognosis. Nevertheless, there are no widely accepted treatment guidelines that have shown significant efficacy or improved benefit in this disease.We report our single institution experience with AITL over the past 10 years.
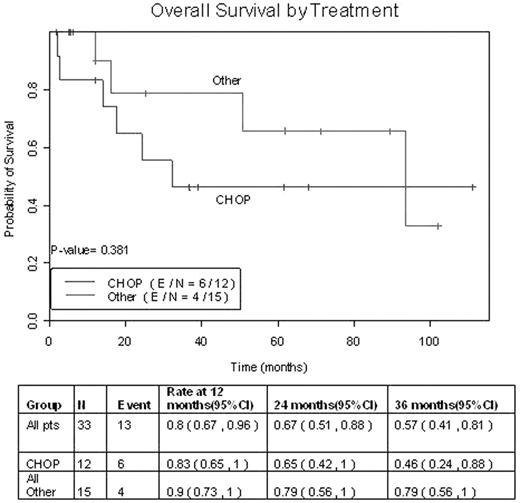
We identified 33 cases of AITL seen at MDACC from 1996–2006. We retrospectively reviewed these cases to analyze clinicopathologic characteristics, treatment regimens, and response rates. The median age at diagnosis was 67 years (43–79), and there was a male to female ratio of 2:1. Thirty-two patients (97%) presented with stage 3 or 4 disease, 16 patients (49%) had bone marrow involvement, and 23 patients (70%) had B symptoms at presentation. Stratifying 32 patients by IPI, 12.5% were classified as low risk, 43.8% as low-intermediate, 28% as high-intermediate, and 15.6% as high risk. A skin rash was present in 16 patients (49%), while 4 patients (12%) had significant inflammatory arthralgias, and 5 patients (15%) had an autoimmune hematologic disorder. Interestingly, 6 patients (18%) had known exposure to industrial chemicals, 8 patients (24%) had a preceding or concurrent second malignancy, and 13 out of the 15 cases (87%) tested for EBV were positive for EBV RNA. Most patients were treated with an anthracycline based regimen. Of these, 6 patients were treated with an intense, alternating triple therapy regimen, 4 patients received a hyperfractionated cyclophosphamide based regimen, and 16 patients received a CHOP-like regimen. Of these 16 patients, 4 also received rituximab in combination with CHOP. Out of 25 evaluable patients after 1st line therapy, 17 patients (68%) achieved CR, 6 patients (24%) achieved PR, and 2 patients (8%) were found to be primary refractory. In those patients with CR, the median duration of 1st remission was 30.5 months (1–96), with a median follow up of 37.6 months (2–101). The probabilities of 1,2, and 3 year survival were 80%, 67%, and 57% respectively. The duration of remission by type of therapy was found to be higher in patients receiving the more intense chemotherapy regimens when compared to CHOP alone. Overall survival of patients treated with CHOP vs. non-CHOP regimens is shown in figure 1. Although the length of followup is still short, notably, only 1 out of 4 patients treated with rituximab have relapsed. The one case had skin-only relapse and has achieved a 2nd remission. The one patient who was treated with upfront high dose chemotherapy and autologous transplant continues to maintain one of the longest durations of remission in our series. Overall response rate and duration of remission in our series was slightly higher than that reported in the literature. Strategies such as early referral to transplant and addition of rituximab to CHOP-like regimens need to be investigated further, as we work to improve the outcome of this disease.
Overall Survival by Treatment
Disclosures: Stock in Genentech.