Abstract
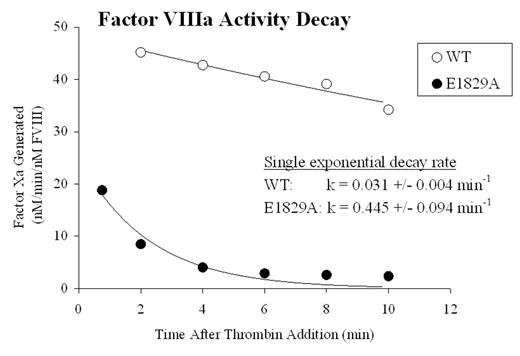
Factor VIII circulates as a heterodimer composed of a heavy chain (HC) and light chain (LC). Thrombin converts factor VIII into the active cofactor, factor VIIIa, by cleaving HC into A1 and A2 subunits. While the A1 subunit maintains a stable interaction with the LC-derived A3C1C2 subunit, the A2 subunit is weakly associated in the trimer and this low affinity interaction accounts for the instability of factor VIIIa activity. Although the majority of binding energy for A2 retention is thought to be derived from its interaction with the A1 subunit, the factor VIII structure model based on homology with ceruloplasmin suggests a binding interface between A2 and A3 domains. While exploring potential calcium binding sites in LC by Ala scanning mutagenesis of acidic amino acids, we encountered one mutant (factor VIII:E1829A) that exhibited an ~10-fold increase in the apparent decay rate of factor VIIIa activity compared with the wild type control, as monitored by a factor Xa generation assay (see figure). This decay rate was significantly reduced by the addition of exogenous A2 subunit. In addition, the activity of factor VIII:E1829A measured by one-stage clotting and two-stage factor Xa generation assays were ~20% and ~10% of wild type factor VIII, respectively, consistent with an assay discrepancy due to increased lability of the mutant factor VIIIa. Affinity values for A1 and A2 subunits for wild type and E1829 A3C1C2 subunits were assessed using subunits purified from the factor VIII proteins. To measure A1 subunit affinity for A3C1C2, wild type or E1829A A3C1C2 subunit (10 nM) was reacted with A1 subunit (0–200 nM), and subsequently factor VIIIa activity from the reconstituted A1/A3C1C2 plus excess A2 subunit (400 nM) was measured by factor Xa generation assay. To measure A2 subunit affinity for A3C1C2, wild type or E1829A A3C1C2 subunit (1 μM) and A1 subunit (100 nM) were reacted and then mixed with A2 (0–1 μM) and regenerated cofactor activity was determined. A1 binding affinity for E1829A-A3C1C2 was comparable to the wild type A3C1C2 (Kd = 20.1 ± 3.4 nM and 15.3 ± 3.7 nM, respectively) while A2 binding affinity was reduced (Kd = 385 ± 54 nM for E1829A, 153 ± 34 nM for wild type). Furthermore, examination of the hemophilia A database shows the mutation of Glu1829 to Gly as a mild phenotype consistent with a functional role for this acidic residue. These results suggest that residue E1829 in the A3 domain contributes to A2 subunit retention following activation of the procofactor, possibly by mediating a binding interaction between the two subunits.
Factor VIIIa Activity Decay
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author