Congenital factor XIII (FXIII) deficiency is associated with a tendency for severe bleeding, a risk for spontaneous abortion, and a high rate of spontaneous intracranial hemorrhage. This phase 1 escalating-dose study was developed to evaluate the safety and pharmacokinetics of a single administration of human recombinant FXIII-A2 (rFXIII-A2) homodimer in adults with congenital FXIII deficiency. Pharmacokinetics and activity of rXIII and changes in endogenous B subunit levels were assessed. Recombinant FXIII-A2 homodimer were complexed with endogenous FXIII-B subunits to form an FXIII-A2B2 heterotetramer with a half-life of 8.5 days, similar to that of endogenous FXIII. The median dose response was a 2.4% increase in FXIII activity based on unit per kilogram rFXIII administered. After the administration of rFXIII-A2, clot solubility normalized as measured by clot lysis in urea. Clot strength and resistance to fibrinolysis, as assessed by thromboelastography, also improved. Safety reviews were conducted before each dose escalation; no serious adverse events, including bleeding or thrombosis, were noted during the study. In addition, there was no evidence of the generation of specific antibodies to rFXIII or yeast proteins. Recombinant FXIII appears to be a safe and potentially effective alternative for FXIII replacement in patients with FXIII deficiency. (Blood. 2006;108:57-62)
Introduction
Factor XIII (FXIII) promotes clot stability by forming intermolecular covalent bonds between fibrin monomers and by cross-linking α-2 antiplasmin, fibrinogen, fibronectin, collagen, and other proteins to enhance the mechanical strength of the fibrin clot, protect from proteolytic degradation, and provide stability to the extracellular matrix.1 Plasma FXIII is a transglutaminase that circulates as a heterotetramer composed of 2 A subunits and 2 B subunits noncovalently linked together and bound to fibrinogen.2,3 The B subunit, which appears to stabilize the structure of the A subunit and to protect the A subunit from proteolysis, is normally present in excess in plasma as free FXIII-B subunit.4,5 FXIII-A subunit homodimers (FXIII-A2) are also found in platelets, chondrocytes, and cells of the monocyte/macrophage lineage.6,7 Persons with congenital FXIII deficiency have a tendency for severe bleeding, a risk for spontaneous abortion, and a high rate (25%-40%) of spontaneous intracranial hemorrhage.8 This high rate of lifethreatening bleeding, combined with the long half-life (8-12 days) of FXIII, makes prophylaxis a viable option for patients with FXIII deficiency, especially those with a history of severe bleeding.9 Deficiency of FXIII is rare. The estimated incidence worldwide is 1 in 1 million to 5 million people.1,10 Most patients with FXIII deficiency have mutations in the FXIII-A subunit; few cases of patients with FXIII-B subunit mutations have been reported in the literature.11-13 Current treatment and prophylaxis options include cryoprecipitate, fresh frozen plasma, and preparations of plasma-derived FXIII concentrates. Placenta-derived FXIII-A2 concentrates are no longer available. Concentrates containing only the FXIII-A2 homodimer (placenta derived) or the FXIII-A2B2 heterotetramer (plasma derived), respectively, have been administered for the treatment and prophylaxis of patients with FXIII deficiency.14,15 A new recombinant FXIII-A2 (rFXIII-A2) homodimer has been manufactured in Saccharomyces cerevesiae and contains no human or mammalian products in the culture or media. The rFXIII-A2 homodimers associate in plasma with the endogenous FXIII-B subunit to form the stable heterotetramer FXIII-A2B2. rFXIII-A2 safety, pharmacokinetics, and immunogenicity have been studied in healthy volunteers.16,17 This phase 1 clinical trial was designed to establish rFXIII-A2 as a safe alternative to human plasma products in the prophylaxis and treatment of FXIII-A2 deficiency through evaluation of the pharmacokinetic and safety profile of a single administration of human rFXIII-A2 in adults with congenital FXIII deficiency.
Patients, materials, and methods
Patient population
This phase 1 escalating-dose study of rFXIII was approved by the Children's Hospital of Orange County Institutional Review Board (IRB) and was funded by ZymoGenetics. Informed consent was provided in accordance with the Declaration of Helsinki. Patients carrying a diagnosis of FXIII deficiency were recruited by referral from physicians in the United States. Inclusion criteria included patients who were at least 18 years of age and had normal platelet counts, prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen levels, renal function, and hepatic function. Exclusion criteria included history of thrombosis or autoimmune disease. Patients were eligible for screening 4 weeks after receiving their last plasma-derived FXIII or blood products. Because of the rarity of this diagnosis, patients were eligible for reentry into the study after 100 days (approximately 10 half-lives) after their last dose of rFXIII. Nine of 11 patients were white, and the median age was 21 years (range, 19-76 years). Six men and 5 women were enrolled (median weight, 77 kg; median height, 170 cm).
Study design
This phase 1 clinical trial was conducted as a single-site, open-label pilot study of rFXIII in patients with congenital FXIII deficiency. Five cohorts, each consisting of 2 patients with FXIII-A subunit deficiency, were to receive 2 U/kg, 6 U/kg, 20 U/kg, 50 U/kg, and 75 U/kg rFXIII. The dose range was selected to increase the plasma FXIII level to 10% to 200%, assuming a starting level of 5% to 25% FXIII activity. The concentration of rFXIII, measured in unit per kilogram, was prepared such that 1 U rFXIII equaled the amount of transglutaminase activity in 1 mL human plasma, and 1 mg rFXIII was equal to 142 U FXIII activity. Previous studies with plasma-derived FXIII-A2 and -A2B2 concentrates showed that concentrations between 10 U/kg and 75 U/kg were well tolerated in patients with FXIII deficiency.14 Between March and September 2003, 11 patients were enrolled. Both patients in the first cohort (20 U/kg) reentered the study at the 75-U/kg cohort because more than 100 days had passed before their second dose of rFXIII. In addition, 3 patients were enrolled at the 50-U/kg dose because one of them had been found to have an FXIII-B subunit deficiency. Recombinant FXIII-A2 was reconstituted to 700 U/mL and was administered through intravenous infusion over 5 minutes. Patients were admitted to the hospital for 72-hour observation and daily clinical and laboratory assessments. Patients in the first 2 cohorts (2 U/kg, 6 U/kg) received their current FXIII replacement therapy after the 72-hour observation period because the rFXIII dose was insufficient for prophylaxis.
Study objectives
Primary objectives were evaluation of the safety of single-dose administration of rFXIII, determination of the pharmacokinetic parameters of rFXIII and effects on endogenous B subunit and FXIII activity, and evaluation of product immunogenicity.
Safety evaluations
Complete blood counts, PT, aPTT, international standardized ratio (INR), fibrin(ogen), and D-dimers were obtained at baseline and at 4 hours, 8 hours, 24 hours, 48 hours, and 72 hours after rFXIII administration and at day 7, day 14, and day 28. Measurements of serum sodium, potassium, chloride, bicarbonate, blood urea nitrogen (BUN), creatinine, glucose, calcium, total bilirubin, cholesterol, AST, ALT, alkaline phosphatase, albumin, total protein, phosphorous, and C-reactive protein were also obtained at screening and at 8 hours, 24 hours, 48 hours, and 72 hours and at day 7, day 14, and day 28. Clinical assessments for deep venous thrombosis were performed at all time points through 72 hours with the use of a deep venous thrombosis (DVT) score chart adapted from Wells et al.18
Pharmacokinetic and pharmacodynamic evaluations
FXIII activity. Blood was collected at the investigative site within 3 days of study drug administration for FXIII activity, clot solubility, and enzyme-linked immunosorbent assay (ELISA) assays. Baseline levels were also determined just before rFXIII administration. Blood was collected at 30 minutes, 1 hour, 4 hours, 8 hours, 24 hours 48 hours, and 72 hours and at day 7, day 14, and day 28 for rFXIII activity, antigen, and clot solubility. Plasma was obtained and stored at -70 C until analysis. FXIII activity was detected using the Berichrom assay (Behringwerke AG, Marburg, Germany) according to the manufacturer's recommendations. The percentage of FXIII activity was calculated against a reference standard of human plasma with 1 U/mL FXIII activity.
Plasma FXIII-A2, FXIII-A2B2, and FXIII-free B subunit levels. All FXIII-A and FXIII-B subunit assays were developed and validated by ZymoGenetics (Seattle, WA), as previously described.16,19 Total A2 (rA2, A2B2,rA2B2) was detected with the use of an ELISA with polyclonal rabbit anti-rFXIII-A2 as the capture antibody and a biotinylated form of the same antibody as the detection reagent. The total A2 assay should in principle quantify each FXIII regardless of its state (A2B2 or A2), but the assay underestimates the quantity of the heterotetrameric form, presumably because of steric hindrance. ELISA to detect native FXIII-A2B2 and rFXIII-A2B2 was performed with polyclonal rabbit anti-human FXIII subunit B as a capture reagent and biotinylated polyclonal rabbit anti-rFXIII-A2 as the detection antibody and only detects the FXIII in the heterotetrameric state. Polyclonal anti-FXIII-B subunit antibody is specific for the B subunit of the tetramer and does not cross-react with the rA2 homodimer (data not shown). FXIII-free B subunit was detected in plasma with an ELISA that uses affinity-purified donkey anti-murine immunoglobulin G (IgG) to capture a monoclonal antibody specific for the FXIII-B subunit. The FXIII-B subunit assay was designed only to detect free FXIII-B subunit, not the FXIII-A2B2 tetramer. However, a small percentage of the tetrameric complex might be detected, either because of crossreactivity with the tetramer (less than 10%) or because of dissociation of the tetramer in highly diluted samples.
Recombinant FXIII and yeast antibody assay
Blood was collected at baseline before rFXIII administration and at the end of study for the rFXIII and yeast antibody assays. An ELISA was developed to detect patient-derived antibodies to rFXIII. rFXIII was used as a capture reagent on the solid phase to bind antibodies from plasma. Protein G-HRP recognizes most IgG subtypes and was used as a detection reagent, followed by streptavidin-HRP and TMB for colorimetric detection of bound antibody from the rabbit anti-rFXIII polyclonal control and from patient samples. If host antibody was detected, antibody titer was established and specificity screening was performed, as previously described,16 to determine whether the antibody was specific for rFXIII. A commercially available modified RAST assay, Allerprint (Esoterix, Austin, TX), which measures allergen-specific IgE, was performed according to the manufacturer's instructions to detect antibody formation to yeast proteins. The assay uses an 8-point calibration curve and the World Health Organization standard for total IgE to quantitate specific antibody levels within patient samples. The assay is sensitive to 0.1 kU/L allergen-specific IgE.
Clot solubility, strength, and stability
Clot solubility was tested using the classic qualitative test of clot solubility in urea at all time points. Blood was collected in 3.2% sodium citrate with a ratio of 9:1. Calcium chloride (0.25 M) was added in a 1:1 ratio to allow clot formation. Once clots were formed, 5 M urea was added and samples were incubated at 37°C. Samples were checked at 15 minutes, 1 hour, and 24 hours. Clots that were not cross-linked by FXIII solubilized within minutes when placed in urea, whereas cross-linked clots were stable for approximately 24 hours. Clot strength and stability were tested with thromboelastography, which provides sequential assessment of clot formation from time to initial clot formation, clot strength, and evaluation of clot breakdown over time.20
Clot initiation, formation, and breakdown were recorded (Haemoscope Thromboelastograph; Haemoscope, Deerfield, IL) at screening and baseline and at 1 hour, 4 hours, 8 hours, 24 hours, 72 hours, and 28 days. Whole blood was collected at all time points with a double-syringe technique. Samples were activated with kaolin and run in duplicate within 4 minutes of collection. Different coagulation parameters were measured. R time (time to initiation of clot formation), K time (clot kinetics), MA (measurement of maximum clot strength), and Ly30 and Ly60 (percentage of clot lysis at 30 and 60 minutes after maximal clot stiffness) were measured at sequential time points, and tracings were obtained for analysis. The shear elastic modulus or G value (dyne/cm2)—a measurement of clot strength and stiffness at each time point—was also calculated.
Safety analysis
An adverse event was defined as any unfavorable or unexpected symptom, sign, or laboratory finding temporally associated with rFXIII infusion regardless of causal relationship. Adverse events recorded for this study were those that occurred within 28 days of rFXIII administration. A serious adverse event was defined as any untoward medical occurrence that resulted in death, that was life threatening and necessitated hospital care or prolonged existing hospital care, or that resulted in persistent or significant disability or incapacity.
Statistical methods
For pharmacodynamic evaluations including dose-normalized response rate, graphical methods were used to assess relationships between dose level and biologic activity markers. Noncompartmental modeling was used to estimate pharmacokinetic parameters for FXIII activity and total FXIII-A2 and FXIII-A2B2 assay data. Pharmacokinetic parameters included initial concentration (Co), area under the curve (AUC), terminal half-life (t½,), clearance (CL), and volume of distribution at steady state (VSS). Pharmacokinetic analysis was performed with WinNonLin 4.0 (Pharmsight). Terminal half-life was determined by linear regression of the terminal portion of the plasma concentration curves from 24 hours to 336 hours. An exception was made for patient 7, who had B subunit deficiency and whose terminal phase was represented from 1 to 24 hours after rFXIII administration.
Results
Pharmacodynamics and pharmacokinetics of rFXIII-A2
Preinfusion FXIII activity measurements were less than 10% of normal (the limit of quantitation of the Berichrom assay [Dade Behring, Marburg, Germany]) in all patients except patient 6, who was ultimately found not to have FXIII deficiency despite a historical diagnosis of this disorder. Pharmacodynamic data using the Berichrom FXIII activity data are presented in Table 1, which shows the percentage increase in FXIII activity for all patients receiving 20, 50, or 75 U/kg rFXIII. Dose-normalized response was calculated, and the median dose-normalized response was 2.4% per U/kg rFXIII (range, 2.1%-3.0% per U/kg rFXIII). Patients in the 2-U/kg and 6-U/kg cohorts received supplemental treatment with plasma-derived FXIII at 72 hours and thus are not included in the pharmacodynamic or pharmacokinetic assessments. Although patient 6 was found not to have FXIII deficiency, the dose-normalized response and baseline corrected pharmacokinetic parameters for this patient were similar to those for the other patients.
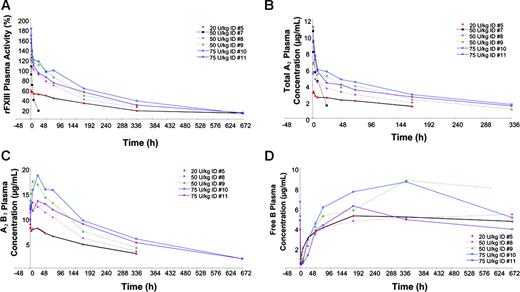
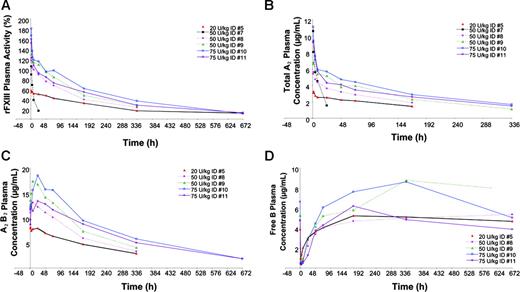
Changes in FXIII activity are graphically presented in Figure 1A. After the administration of 20 U/kg, 50 U/kg, and 75 U/kg rFXIII, patients showed FXIII plasma activity increases of 57% to 59%, 105% to 129%, and 160% to 181%, respectively. Pharmacokinetic data are shown in Table 2. Terminal half-life (t½) ranged from 149 to 217 hours (6.2-9.0 days) except in patient 7 with FXIII-B subunit deficiency, whose t½ was 8.9 hours. Initial concentration (Co) increased in a dose-proportional manner, from 3.8 μg/mL to 4.7 μg/mL in the 20-U/kg rFXIII group, 8.2 μg/mL to 9.1 μg/mL in the 50-U/kg rFXIII group, and from 11.7 μg/mL to 13.6 μg/mL in the 75-U/kg rFXIII group. Area under the curve (AUC) also increased in a dose-dependent manner, and clearance (0.10-0.25 mL/h per kilogram) was consistent across dose groups except in the patient with FXIII-B subunit deficiency, whose clearance was faster (4.28 mL/h per kilogram). The mean volume of distribution (VSS) at steady state ranged from 41.0 to 76.1 mL/kg, indicating that rFXIII-A2 remains primarily in the plasma volume estimated to be approximately 43 mL/kg per 70-kg person.
Plasma FXIII-A2, FXIII-A2B2, and FXIII-free B subunit pharmacokinetics in patients with FXIII-A subunit deficiency
Total FXIII-A2 and FXIII-A2B2 immunoassays yielded similar pharmacokinetic parameters compared with FXIII activity for patients in the 3 highest dose cohorts (data not shown). Changes in plasma FXIII-A2, FXIII-A2B2, and FXIII-B subunit levels after infusion of rFXIII are shown in Figure 1. Interestingly, there was a steady increase in FXIII-A2B2 levels in the first 24 hours, suggesting that the endogenous FXIII-B subunit was released into circulation within the first 24 hours of administration and was bound with residual free rFXIII-A2.
FXIII-free B subunit concentrations decreased rapidly after administration, then gradually increased in the first 36 to 72 hours and eventually returned to predose levels, as shown in Figure 1D. This finding supports the hypothesis that recombinant FXIII-A2 rapidly combines with the endogenous FXIII-B subunits to form the stable rFXIII-A2B2 heterotetramer.
Plasma FXIII-A2, FXIII-A2B2, and FXIII-B subunit pharmacokinetics in a patient with FXIII-B subunit deficiency
Patient 7 was enrolled in the 50-U/kg cohort and was found during pharmacokinetic analysis to have FXIII-B subunit deficiency. This patient was diagnosed with FXIII deficiency in 1999 after a long history of menorrhagia and postsurgical bleeding. As shown in Table 2, her pharmacokinetic profile was markedly different from that of the 2 patients with FXIII-A2 deficiency who were enrolled in the same dose cohort. The rFXIII pharmacokinetic profile in this patient showed a shortened terminal half-life of 8.9 hours, with markedly decreased AUC and increased clearance compared with the patients with FXIII-A2 deficiency. FXIII-A2B2 and FXIII-B subunit levels were undetectable at all time points.
Urea clot solubility and thromboelastography
Clot solubility was measured using the qualitative urea clot solubility method, and clot strength was measured by thromboelastography. Abnormal clot solubility in urea at baseline was found in all patients with FXIII-A subunit deficiency. In patients who received at least 20 U/kg rFXIII, normal urea clot solubility was restored for the 28-day period of the study. The patient with FXIII-B subunit deficiency had normal urea clot solubility test results at screening but not at baseline (both before rFXIII administration). Clot solubility normalized at 24 and 48 hours but was abnormal 72 hours after rFXIII administration. Urea clot solubility reverted to normal without further treatment, suggesting that low levels of endogenous circulating FXIII-A2 in this patient with FXIII-B deficiency might have undergone sufficient FXIII cross-linking activity to be at the limit of detection for this qualitative assay.
Activity and concentrations of FXIII after infusion of 20, 50, or 75 U/kg rFXIII in patients with FXIII deficiency. (A) Individual FXIII plasma activity. (B) Individual total FXIII-A2 plasma concentrations. (C) Individual FXIIIA2 B2 heterotetramer concentrations. (D) Individual FXIII-free B subunit concentrations.
Activity and concentrations of FXIII after infusion of 20, 50, or 75 U/kg rFXIII in patients with FXIII deficiency. (A) Individual FXIII plasma activity. (B) Individual total FXIII-A2 plasma concentrations. (C) Individual FXIIIA2 B2 heterotetramer concentrations. (D) Individual FXIII-free B subunit concentrations.
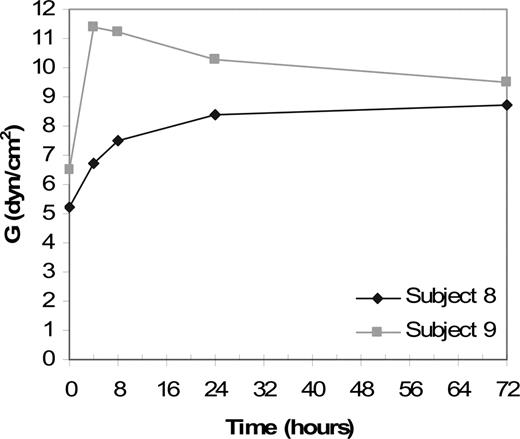
Clot strength and resistance to fibrinolysis were also measured by thromboelastography. Baseline tracings from patients with FXIII-A deficiency showed decreased MA and G and increased Ly60 compared with tracings from healthy patients (data not shown). Thromboelastography data from patients receiving 50 U/kg rFXIII-A2 are shown in Figure 2. A representative thromboelastography tracing from patient 8, who received 50 U/kg rFXIII-A2, is shown in Figure 3. The MA, which indicates clot strength, increased from 50.8 mm at baseline to 63.4 mm at 72 hours and then decreased to 58.4 mm at day 28, indicating that a stronger clot was formed after rFXIII infusion. Ly30 and Ly60 values, which indicate the percentage of lysis at 30 and 60 minutes after clot formation, both showed improvement after rFXIII infusion. For example, Ly60 for patient 8, who received 50 U/kg rFXIII, was 12.8% at baseline, decreased to 4.8% at 72 hours, and increased to 8.6% at the end of the study. Lower values for Ly30 and Ly60 after rFXIII infusion indicated that the clot was more resistant to fibrinolysis. Clot strength and resistance to fibrinolysis in whole blood, as measured by thromboelastography, showed significant changes at therapeutic doses that increased the FXIII level by at least 50% (data not shown).
Immunogenicity evaluations
Antibodies to FXIII and yeast were assayed at screening and at day 28. Two patients, patient 3 and patient 6, had preexisting detectable antibodies to FXIII that remained unchanged during the study. Samples from these 2 patients were further assayed to evaluate specificity, and the antibody response was nonspecific for rFXIII before and after infusion. Yeast antibody formation was also analyzed and remained clinically insignificant throughout the study. All posttreatment titers were lower than 0.11 kU/L except the titer for patient 10, which was 0.2 kU/L.
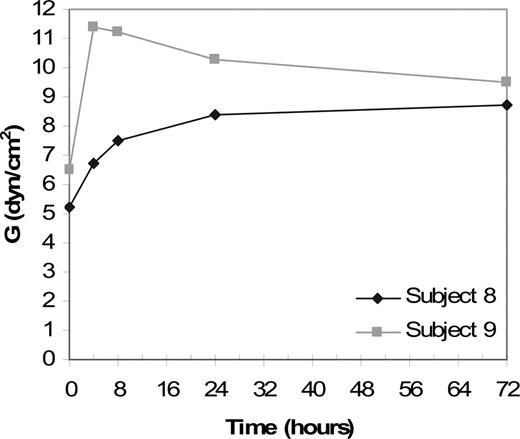
Thromboelastography results for patient 8 and patient 9. Clot strength over time for patient 8 (♦) and patient 9 (▦). Patients received 50 U/kg rFXIII at time point 0. Clot strength (G) is shown at 4, 8, 24, and 72 hours. Clot strength, as expressed by G values in dyne/cm2, increased after rFXIII infusion and remained above baseline for 72 hours.
Thromboelastography results for patient 8 and patient 9. Clot strength over time for patient 8 (♦) and patient 9 (▦). Patients received 50 U/kg rFXIII at time point 0. Clot strength (G) is shown at 4, 8, 24, and 72 hours. Clot strength, as expressed by G values in dyne/cm2, increased after rFXIII infusion and remained above baseline for 72 hours.
Safety analysis
No serious adverse events, including thrombosis, were reported. Eleven adverse events were reported in 4 patients, with patient 4 experiencing 6 of these (Table 3). The most common adverse event reported was headache, which occurred in 3 (27.3%) patients; the next most common was arthralgia, reported in 2 patients. Patient 4 was injured playing softball and developed pain, swelling, and a small contusion on her ankle 5 days after receiving rFXIII. Patient 7, who had B-subunit deficiency, reported body aches 7 days after rFXIII administration, but they resolved without treatment. Adverse events did not appear related to the dose or timing of rFXIII administration.
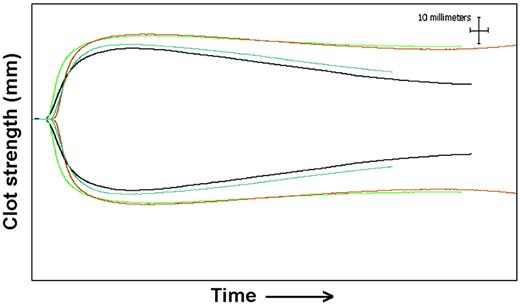
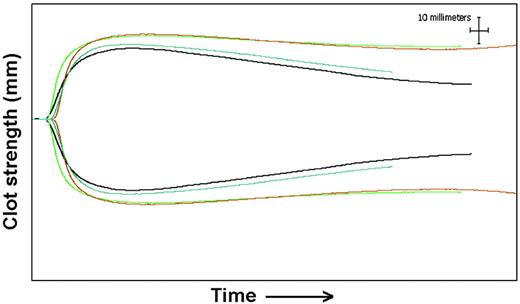
Thromboelastography results for patient 8. Baseline tracing (black) before administration of 50 U/kg rFXIII. Tracings at 28 days (blue) show decreased clot strength (MA) and increased fibrinolysis (increased percentage of Ly60). Tracings from 24 hours (green) and 72 hours (red) show increased clot strength and resistance to fibrinolysis. The x-axis shows time from the addition of sample to thromboelastography, and the y-axis shows distance in millimeters, which reflects clot strength.
Thromboelastography results for patient 8. Baseline tracing (black) before administration of 50 U/kg rFXIII. Tracings at 28 days (blue) show decreased clot strength (MA) and increased fibrinolysis (increased percentage of Ly60). Tracings from 24 hours (green) and 72 hours (red) show increased clot strength and resistance to fibrinolysis. The x-axis shows time from the addition of sample to thromboelastography, and the y-axis shows distance in millimeters, which reflects clot strength.
No clinically significant changes were observed in complete blood counts, chemistry profiles, or coagulation assays after rFXIII infusion. AST and ALT liver enzyme levels increased modestly in one patient in the 20-U/kg group, but they returned to normal; increased levels were not observed in patients receiving the higher doses.
Discussion
This study demonstrates that rFXIII-A2, when combined with endogenous FXIII-B subunits, has a half-life similar to that of native FXIII and appears to be safe and to result in no serious adverse events in patients with FXIII deficiency. No increase in adverse events was reported with dose escalation, and one patient in the second cohort experienced more than half the adverse events reported. In addition, no adverse events were thought to be temporally related to drug infusion. We saw no evidence of increased production of antibody to FXIII, in agreement with the results of previous studies in patients with congenital FXIII deficiency receiving long-term administration of plasma-derived, FXIII-containing products.21 Additionally, 2 recently completed studies16,17 of rFXIII-A2 in healthy volunteers demonstrated no antibody response to yeast or rFXIII in any patients at any of the doses tested, suggesting that the recombinant molecule is not immunogenic.
Recombinant FXIII-A2 combined with endogenous FXIII-B subunits had pharmacokinetics similar to those of native FXIII, suggesting that monthly administration of rFXIII may be used as prophylaxis for patients with FXIII-A subunit deficiency. The half-life of rFXIII-A2 combined with FXIII-B was determined to be 8.5 days, with a median dose response of 2.4% increase in FXIII activity for every unit of rFXIII per kilogram administered. The previous study16 with single-dose recombinant FXIII-A2 at 50 U/kg in healthy volunteers showed a similar half-life of 12.2 days and a median dose response of 1.7% increase in FXIII activity for every unit of rFXIII per kilogram administered. These values are similar to the reported median half-life of 9.1 days and a median dose response of 1.5% increase in activity for every unit of placenta-derived FXIII-A2 per kilogram reported by Brackman et al.22 In comparison, the patient with FXIII-B subunit deficiency had a half-life of approximately 9 hours after rFXIII-A2 infusion. Previous studies of placenta-derived FXIII-A2 also demonstrated similar pharmacokinetics in a patient with FXIII-B subunit deficiency.23 The immediate decrease in free B subunit levels after rFXIII-A2 infusion suggests that rFXIII-A2 complexes with the FXIII-B subunits in plasma. The reduced half-life of rFXIII-A2 in the absence of native FXIII-B subunit further supports the concept that the FXIII-B subunit serves as a protective carrier molecule.
Little is known about the regulation of either the FXIII-A2 or the FXIII-B subunit in circulation. Previous investigators have suggested that the level of FXIII-A2 subunit may have a regulatory effect on the FXIII-B subunit concentration.24 The main site of production of the circulating FXIII-A2 subunits is still debated because there is evidence for synthesis in the liver and the bone marrow.25,26 The liver is thought to be the site of synthesis of the FXIII-B subunit.27 At the 3 highest rFXIII-A2 doses administered, after the initial decrease in FXIII-B subunit levels, a steady increase in newly formed rFXIII-A2B2 was observed in the first 24 hours. This finding suggests that de novo synthesis or release of FXIII-B subunits into the circulation occurs in response to circulating levels of free FXIII-A2. It is clear that additional studies are needed to arrive at an understanding of this complex regulatory process.
Changes in clot strength and stability were shown using urea clot solubility and thromboelastography. All patients with FXIII-A subunit deficiency had abnormal urea clot solubility values at baseline. However, after infusion of at least 20 U/kg rFXIII, clot solubility normalized for the 28 days of the trial, suggesting that monthly prophylaxis with rFXIII-A2 concentrate is a viable option for patients with FXIII-A subunit deficiency. Thromboelastography results in whole blood demonstrated increased clot strength and resistance to fibrinolysis at higher doses of rFXIII-A2. Similar findings have been reported with an in vitro addition of 10 μg/mL rFXIII-A2 to blood taken from patients who underwent cardiac bypass surgery.28 The amount of rFXIII-A2 added in vitro in that study corresponded to approximately 20 U/kg in a 70-kg adult. We believe thromboelastography may have a role in monitoring response to FXIII replacement; however, additional studies are needed to evaluate our methodology.
In summary, results from our study indicate the rFXIII may be a safe and effective alternative to plasma-derived products in patients with FXIII-A2 deficiency. Pharmacodynamic and pharmacokinetic data, in addition to our good initial safety profile, support monthly administration of 20 U/kg to 75 U/kg as possible prophylaxis regimens for patients with FXIII-A2 deficiency. Further studies are required to optimize dosing and to confirm safety and immunogenicity over a longer study period. Recombinant FXIII-A2 may also be useful in patients with acquired FXIII deficiency, including those who have undergone cardiac bypass surgery28 and those who have graft-versushost disease,29 inflammatory bowel disease,30 and disturbed endothelial cell barrier function.31
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-02-0788.
Supported by research funding from ZymoGenetics, Inc.
Several of the authors (T.R., J.V., M.D.B., S.P., L.I.) are or were employed by ZymoGenetics, whose potential product was studied in this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the physicians who referred their patients for enrollment, including Dr Juan Chediak, Dr Rafael Ducos, Dr Robert Janco, Dr Peter Marks, Dr Jamie Siegel, Dr Arthur Thompson, and Dr Mark Heiny, and we thank Kathie Birschbach and Vicki Giraldin for secretarial assistance.