Overt stroke, clinically “silent” cerebral infarct, and neurocognitive impairment are frequent complications of sickle cell anemia (SCA). Current imaging techniques have limited sensitivity and specificity to identify children at risk for neurocognitive impairment. We prospectively evaluated 24 children with SCA with a neurologic exam, complete blood count, transcranial Doppler ultrasound (TCD), measurement of intelligence quotient (IQ), and magnetic resonance imaging (MRI) with measurement of cerebral blood flow (CBF) using continuous arterial spin-labeling (CASL) MRI. Average CBF to gray matter was 112 ± 36 mL/100 g/min. We identified a strong inverse relationship between performance IQ and CBF (-1.5 points per 10 mL/100 g/min increase in CBF, P = .013). Elevated steady-state white blood cell count (≥ 14 × 109/L [14 000/μL]) was associated with lower full scale IQ (86 ± 9 vs 99 ± 10, P = .005). CASL MRI may identify children with neurocognitive impairment, before damage is evident by structural MRI or TCD. (Blood. 2006;108:379-381)
Introduction
Neurologic complications are common in children with sickle cell anemia (SCA); by age 18, 11% will have had an overt stroke and an additional 15% to 22% will have had a silent cerebral infarct (SCI).1-3 On average, children with SCA and magnetic resonance imaging (MRI)-confirmed SCI have lower intelligence quotients (IQs) than those with a normal MRI.4 Furthermore, even those with normal MRIs perform worse on neuropsychologic testing than their unaffected siblings.3 Possible etiologies include effects of vaso-occlusion or anemia on the developing brain. Low hematocrit (< 20%) and elevated platelet count (>500 × 109/L [500 000/μL]) are also associated with decreased IQ in children with SCA.3,5 Cerebral blood flow (CBF) may also affect neurocognitive function. Children with SCA and abnormal CBF velocity, measured by transcranial Doppler ultrasound (TCD), perform more poorly on tests of verbal intelligence and executive function.6 We examined the relationship between CBF and neurocognitive function in children with SCA and a normal TCD. We hypothesized that measuring CBF by MRI could identify children at increased risk for neurocognitive impairment and SCI. These children could then be targeted for educational and other specific interventions.
Study design
Study population
We prospectively enrolled children aged 6 to 12 years with homozygous sickle cell disease (HbSS) or sickle β-null thalassemia from our pediatric hematology clinic. We excluded children with previous traumatic brain injury, seizure disorders, abnormal TCDs (> 200 cm/sec), stroke, other identified causes of cognitive impairment, and those receiving scheduled transfusions.
Study procedures
At the initial study visit, children had a standardized exam by a pediatric neurologist, a TCD, a brain MRI at 1.5 Tesla, neuropsychologic testing, and venous blood collected. All testing, except for the neurologic exam, was repeated at 12-18 months follow-up. TCD studies were performed with a 2-MHz pulsed ultrasound probe (Intraview; RIMED, Raanana, Israel) per published methods.7-9 MRIs (fluid-attenuated inversion recovery [FLAIR], transverse relaxation time [T2], diffusion-weighted sequences, and MR angiography) were assessed by 2 neuroradiologists for the presence of SCI (T2 or FLAIR hyperintensities ≥ 3 mm in diameter consistent with ischemia and seen in 2 planes). IQ was measured by a licensed neuropsychologist using established methods.10 The neurologists, neuroradiologists, and neuropsychologist were masked to the results of other studies. CBF was measured using continuous arterial spin-labeling (CASL) MRI, a technique that labels water molecules in blood by a radiofrequency pulse as they pass through a plane in the upper neck. Labeled images are subtracted from control (unlabeled) images to estimate CBF. We obtained and processed the images per Oguz et al, but substituted specific values for the spin-lattice relaxation time (T1) of blood at 1.5 T based on packed-cell volume (PCV).11,12
The vascular distributions of the major cerebral arteries13 were independently outlined by 2 investigators and used to calculate regional CBF to gray matter. Blood studies, including PCV, white blood cell (WBC) count, and platelet count, were performed using an automated analyzer (Sysmex SE 9500; Roche, Indianapolis, IN) when the patients were in their usual state of health. The study was approved by the Johns Hopkins Medicine Institutional Review Board, and we obtained consent from the parent/guardian and assent from the child.
Statistical analysis
We converted CBF velocity to standardized values. We calculated the Pearson correlation coefficient and compared continuous variables between groups with the Student t test. We used linear regression with adjustment for clustering to evaluate the relationship between CBF velocity and IQ. We examined the relationship between CBF, baseline patient characteristics, PCV, WBC count, platelet count, and IQ using the same techniques.14
Results and discussion
We studied 24 children from July 2000 to January 2004. Seventeen returned for their scheduled follow-up visit at 12 to 18 months, and 4 also returned at 6 months (Table 1). All children had SCA, and 2 were taking hydroxyurea. Their mean age at the first visit was 8.5 ± 2.0 years. Five of the 21 children had mild abnormalities detected by neurologic exam, including residual left facial nerve palsy after a documented Bell palsy (1), right carotid bruit (1), subtle pyramidal deficits (2), and mild cerebellar signs (1), without corresponding abnormalities on imaging. Ten children had 1 or more of the following: abnormalities of attention (7), received special services in school (6), or repeated a year of school (5). Only 1 of 36 TCDs was conditional by established standards for SCA.15 Of 24 children with 38 interpretable MRIs and MRAs, 1 child had SCI and irregularity at the trifurcation of the left middle cerebral artery (MCA) on 3 studies and another had a mild stenosis of the M2 segment of the MCA on 2 studies.
Mean CBF in the distribution of the right and left anterior cerebral arteries was 108 ± 40 mL/100 g/min and 111 ± 38 mL/100 g/min, and was similar for the middle and posterior cerebral arteries. The 10 children with abnormalities of attention or school difficulty had lower full-scale IQ (91 ± 12 vs 101 ± 12, P = .05) than those without this history. We found no significant correlations between standardized CBF velocity and CBF by region (r =-0.13-0.14, P > .5 for all comparisons) or between CBF velocity and IQ (r = 0.04, P = .82 for full scale IQ). The poor correlation between regional CBF and CBF velocity of the major arteries could reflect the contribution of other vessels through the Circle of Willis.
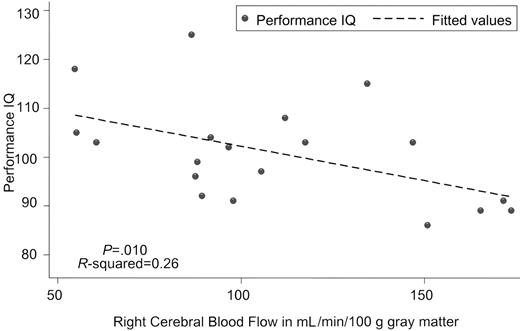
Kral and Brown16 also reported poor correlation between CBF velocity and full scale IQ (r =-0.08), although those with abnormal TCDs did have lower verbal IQs. In contrast, we identified a strong inverse correlation between CBF and both full scale (Table 1) and performance IQ (Figure 1), which suggests that CBF is a more sensitive indicator than CBF velocity. The relationship was strongest between performance IQ and anterior and right-sided CBF. These brain areas are essential for the organizational and visual spatial skills used in block design and matrix reasoning, 2 subtests of performance IQ.10 Overall, regional CBF was highly correlated (r = 0.87-0.99) which explains the similar relationship between CBF by region and IQ.
Scatter graph of performance intelligence quotient versus right cerebral blood flow to gray matter with fitted values estimated by linear regression.
Scatter graph of performance intelligence quotient versus right cerebral blood flow to gray matter with fitted values estimated by linear regression.
Children with WBC counts in the highest quartile (≥ 14 × 109/L [14 000/μL]) had significantly lower full scale IQ (86 ± 9 vs 99 ± 10, P = .005), whereas children with low PCV (< 20%) or elevated platelet count (≥ 500 × 109/L [500 000/μL]) did not. To our knowledge, an association between WBC count and IQ has not been described previously, although increased WBC count at baseline was associated with increased risk of early death in a large cohort study of SCA.17 WBC count may serve as a marker of inflammatory mediators, which have been associated with increased risk of stroke and cognitive impairment in adults,18 or of decreased splenic function and severe disease in SCA.1
Studies using qualitative interpretation of positron emission tomography (PET)19 or quantitative MRI suggest that children with decreased regional CBF or low T1 values20 have lower IQs. However, the inclusion of patients with stroke and SCI in the PET study and of patients with sickle hemoglobin C disease (HbSC) in the MRI study may have confounded those results. Our quantitative results suggest that there may be an inverse correlation between regional CBF and IQ. This relationship was strongest for performance IQ, which may reflect sensitivity to global oxygen delivery. We postulate that increased CBF is both a response to and a risk factor for cerebral hypoxia. Prohovnik et al21 demonstrated increased CBF in a cohort of patients with SCD more than 15 years ago using 123Xenon inhalation. They proposed that increased CBF resulted from adaptive vasodilatation and that it caused a reduction in cerebrovascular reserve. Further demand (eg, fever) or decrease in oxygen delivery (eg, worsening anemia or a drop in perfusion pressure) in patients with the high CBF could cause distal infarction. The increase in CBF in their report was greater than that expected based on the severity of anemia, and decreased more than expected after transfusion with sickle-negative blood.21 This implies that hemoglobin S has an independent effect on CBF. Increased CBF may result from abnormal vasoregulation secondary to the effects of hemolysis and free hemoglobin on nitric oxide.22
Limitations of this study include small sample size, convenience sampling, and limited longitudinal measurement of CBF. Our sample of patients, while not deliberately selected for these characteristics, had a lower prevalence of SCI and conditional TCDs and, on average, higher IQ than the general population of children with HbSS. However, it is striking that we still found an inverse correlation between CBF and IQ that was not explained by SCI or worsening anemia. CASL MRI can be performed successfully without sedation and will become more sensitive and more available to clinical practice with the advent of higher magnetic fields (3 T). Measurements of CBF by CASL at 1.5 T are reproducible (coefficient of variation of 6% for measurements repeated the same day)23 and correlate well with PET studies of CBF in a cortical strip (64 ± 12 mL/100 g/min vs 67 ± 13 mL/100 g/min).24 Our results suggest that globally increased CBF, perhaps preceding focal stenosis, may be a risk factor for CNS complications. CASL MRI is a promising modality that allows measurement of CBF noninvasively and without exposure to radiation. CASL MRI, by detecting increased CBF, may allow interventions to modify the risk of neurocognitive impairment from SCA, potentially before SCI or abnormal CBF velocity develop. Larger studies with additional follow-up are necessary to validate the results of this pilot study, and to further evaluate CASL MRI.
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2005-10-4029.
Supported by the National Institutes of Health/National Cancer Institute Clinical Research Training Grant 5K12CA01709-12 (J.J.S.). Supported in part by the General Clinical Research Center (National Institutes of Health/National Center for Research Resources [NIH/NCRR] grant M01-RR000052) at the Johns Hopkins University School of Medicine and the National Institute of Neurological Diseases and Stroke grant 5R21NS040035-02.
One of the authors, (P.C.v.2.) has declared a financial interest in a company, Philips Medical Systems, whose product, ACS NT Power Trak 6000 MRI Scanner, was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.