Efficient and quick reconstitution of T-cell compartments in lymphopenic patients is of great importance to prevent opportunistic infections, but remains difficult to achieve. Human T-cell proliferation in a T-cell-receptor (TCR)-independent manner is possible in vitro with superagonist anti-CD28 antibodies, and such molecules are therefore promising therapeutic tools. Here, we investigated the in vivo effects of superagonist anti-CD28 treatment on human developing and mature T cells, in the recently developed model of “human immune system” BALB/c Rag2-/-γc-/- mice. Our results show that superagonist anti-CD28 treatment transiently induces a 7-fold increase in thymocyte numbers and up to 18-fold accumulation of mature thymocytes. The increased thymic production lead to transient accumulation of mature T cells in the periphery at the peak of treatment effect (day 6). In addition, long-term peripheral T-cell depletion was induced. Furthermore, the concomitant selective expansion and accumulation of suppressive CD4+CD25+FoxP3+ T cells was induced in a transient manner. Superagonist anti-CD28 therapy could therefore be of clinical interest in humans, both for beneficial effect on thymic T-cell production as well as regulatory T-cell accumulation. (Blood. 2006;108:238-245)
Introduction
T-cell lymphopenia is a common side effect in patients undergoing autologous and allogenic hematopoietic stem cell (HSC) transplantation, increasing the risk of opportunistic infections.1,2 The reconstitution of the peripheral T-cell compartments depends on 3 major processes: (1) de novo production of naive T cells in the thymus, with an age-dependent decline due to thymic involution3 ; (2) slow lymphopenia-driven proliferation of peripheral T cells under the control of environmental T-cell receptor (TCR) cross-reactivity and cytokines, such as interleukin (IL)-7 and IL-154,5 ; and (3) antigen-driven clonal expansion. However, lymphopenia-driven proliferation and immune responses intrinsically narrow the available T-cell repertoire, and this can lead to the outgrowth of clones highly cross-reactive with self-antigens during a phase of lymphopenia (ie, when T-cell competition for resources like major histocompatibility complex [MHC] molecules and cytokines is reduced).4,6,7 Skewing of the T-cell repertoire is also observed in mouse bone marrow (BM) chimeras reconstituted with limited numbers of immunocompetent T-cell precursors.8 Such a situation can lead to disruption of T-cell homeostasis, development of autoimmune diseases, and limitation of immune responses to new antigens.9 Therefore, it appears to be of major importance that intensive de novo T-cell production by a functional thymus occurs during such a period of T-cell recovery.
Proper activation of naive T cells relies on surface engagement of both the TCR and costimulatory molecules, and the latter are essential to avoid induction of unresponsiveness of the stimulated T cells.10 Among the large amount of known costimulatory molecules, CD28 and its ligands of the B7 family have been extensively studied.11,12 CD28 triggering is most effective on naive and resting T cells, and induces the expression of CD40 ligand (CD40L) on their surface. Its role in the formation of the immunologic synapse13,14 reinforces the idea that CD28 costimulation participates in the decrease of the threshold of T-cell activation.15 Furthermore, in addition to promoting optimal T-cell activation, CD28 ligation also prevents T-cell anergy and apoptosis.10,16-18 Indeed, polyclonal expansion of T cells can be achieved in vitro by using immobilized monoclonal antibodies to the TCR and CD28.19 Such an approach has failed in vivo, mainly because the use of anti-TCR complex antibodies leads to the release of proapoptotic and proinflammatory cytokines.20,21 Superagonist (SA) CD28-specific antibodies have the unique properties of inducing T-cell activation independent of TCR engagement,22-24 and such antibodies may be applied as an alternative for anti-CD3/TCR antibodies. Several promising results of SA anti-CD28 treatment have been described in the rat, specifically on T-cell proliferation (both in vitro and in vivo), and regulatory CD4+CD25+ T-cell subset expansion.25-27 Overall, these observations suggest that SA anti-CD28 treatment could be used in patients as a tool to improve the recovery from T-cell lymphopenia,24 with specific effects on the CD4+CD25+ T-cell pool.
We and others have recently established humanized BALB/c Rag2-/-γc-/- mice as a new xenograft model for the in vivo study of development and function of the human immune system (HIS).28,29 In this HIS (BALB-Rag/γ) mouse model, development of all major human myeloid and lymphoid cellular compartments, including T cells, takes place with high efficiency compared with previously available models.30 HIS (BALB-Rag/γ) mice therefore represent an interesting opportunity to investigate the in vivo influence of SA anti-CD28 therapy on human thymocytes and their mature progeny. In this study, we show that treatment with SA anti-CD28 induces within a week a transient accumulation of mature human T cells in the thymus. Furthermore, suppressive human CD4+CD25+FoxP3+ T cells also accumulate transiently in the thymus and the lymphoid organs of treated animals. In addition, the SA anti-CD28 treatment induced long-term peripheral T-cell depletion. Our results therefore indicate that SA anti-CD28 therapy enhances the production of mature human T cells by the thymus and notably of human Treg cells in HIS (BALB-Rag/γ) mice, which could be beneficial for patients suffering from autoimmune disorders.
Materials and methods
Generation of HIS (BALB-Rag/γ) mice
H-2dRag2-/-γc-/- mice31 were bred and maintained in isolators, and were fed autoclaved food and water. Mice with a human immune system (HIS [BALB-Rag/γ]) were generated as previously described.28 Briefly, newborn (< 1week old) Rag2-/-γc-/- mice received sublethal (3.5 Gy) total body irradiation with an x-ray Röntgen source, and were injected intraperitoneally with 0.5 to 1 × 106 CD34+ human fetal liver cells. Magnetic enrichment of CD34+ cells (> 98% pure) was performed by using the CD34 Progenitor Cell Isolation Kit (Miltenyi Biotech, Auburn, CA), after preparation of single-cell suspension and isolation of mononuclear cells by density gradient centrifugation over Lymphoprep Ficoll-Hypaque (Nycomed Pharma, Roskilde, Denmark). Human fetal liver was obtained from elective abortions, with gestational age ranging from 14 to 20 weeks. The use of this tissue was approved by the Medical Ethical Committee of the AMC-UvA and was contingent on informed consent. All manipulations of HIS (BALB-Rag/γ) mice were performed under laminar flow. Cell suspensions were prepared in RPMI medium with 2% fetal calf serum.
Treatment with SA anti-CD28 antibody
The mouse immunoglobulin G1 (IgG1) clone 5.11A1 monoclonal antibody (mAb) was previously described as being a superagonist antibody specific for human CD28.23 Treatment procedure was adapted from the protocol used in rats26 as follows: reconstituted (> 10% huCD45+ mononuclear cells in blood) adult (> 8 weeks after HSC inoculation) HIS (BALB-Rag/γ) mice were injected once intraperitoneally with phosphate-buffered saline (PBS)-diluted 5.11A1 mAb ranging from 0.3 mg to 0.03 mg, in comparison to 1 mg routinely applied to rats. In some experiments, young animals (around 4 weeks after reconstitution) were treated with 0.3 mg of mAb. Control animals were treated with isotype control mouse IgG1 antibody (Sigma, St Louis, MO).
Flow cytometry analysis for cell-surface and intracellular markers
Cell suspensions were labeled with antihuman mAb targeting the following cell-surface markers: CD1a (T6-RD1) from Beckman Coulter (Fullerton, CA), CD3 (SK7), CD4 (SK3), CD8 (SK1), CD19 (HIB19), CD25/IL-2Rα (2A3), CD28 (L293), CD45 (2D1), CD45RO (UCHL-1), CD69 (L78), and CD123/IL-3Rα (9F5) from BD Bioscience (San Jose, CA); CD27 (LT27) from Serotec (Raleigh, NC), BDCA2 (AC144) from Miltenyi Biotech; and GITR/TNFRSF18 (110416) from R&D Systems (Minneapolis, MN).
The expression of the transcription factor FoxP3 was analyzed by intracellular (ic) staining with phycoerythrin (PE)-coupled anti-human FoxP3 mAb (PCH101; eBioScience, San Diego, CA) after fixation and permeabilization of the cellular suspensions using the reagents provided by the manufacturer. Lymphocyte turnover was analyzed in vivo after 2 injections of 0.8 mg bromodeoxyuridine (BrdU) intraperitoneally with a 12-hour interval. One day after the first injection, harvested cells were analyzed as previously described,32 using an PE-coupled anti-BrdU mAb (3D4; BD Bioscience). Apoptotic cells were analyzed by labeling with Annexin-V (BD Bioscience) and 7-AAD (Calbiochem, San Diego, CA). All washings and reagent dilutions were done with PBS containing 2% fetal calf serum (FCS) and 0.02% sodium azide (NaN3). All acquisitions were performed with a LSR-II (BD Bioscience) cytometer interfaced to the fluorescence-activated cell sorting (FACS)-Diva software system (BD Bioscience).
Cell sorting for FOXP3 mRNA analysis and suppression assay
Mature human CD4+CD25+ and CD25- T cells were sorted (≥ 99% pure) from spleens and thymi of HIS (BALB-Rag/γ) mice using an ARIA sorter (BD Bioscience). The mRNA was isolated from 5 × 103 to × 105 sorted cells and reverse transcribed using standard protocols. The following primers were used for polymerase chain reaction (PCR) on cDNA: FOXP3 Fw 5′-GAA AAT GGC ACT GAC CAA GG and Rev 5′-GAG GAA CTC TGG GAA TGT GC; and huACTIN Fw 5′-CAA GAG ATG GCC ACG GCT GCT TCC AGC and Rev 5′-ATG GAG TTG AAG GTA GTT TCG. Cells sorted from the thymus of SA anti-CD28-treated HIS (BALB-Rag/γ) mice were also analyzed for their suppressive capacity in 96-well plates, as previously described.33 In brief, 15 to 50 × 103 sorted cells (CD45+CD3+CD1a-CD8-CD4+CD25high vs CD25- thymocytes) were cultured alone or mixed (1:1 ratio) in complete RPMI medium supplemented with 10% normal human serum and antibiotics, in presence of 0.4 μg/mL anti-CD3 mAb (PeliCluster; Sanquin, Amsterdam, The Netherlands) and 50 × 103 irradiated (20 Gy) human peripheral-blood mononuclear cells. After 3 days of culture, each well received 0.0296 MBq (0.8 μCi) 3H-thymidine for overnight incubation before harvesting and measurement of tritium counts.
Statistical analyses
Data were subjected to 2-tail unpaired Student t test analysis where indicated in the figure legends. The obtained P values were considered significant when less than .05.
Results
T-cell development in HIS (BALB-Rag/γ) mice
A fully differentiated multilineage human immune system can be reconstituted in vivo by injecting newborn alymphoid BALB/c Rag2-/-γc-/- mice with human CD34+ progenitors.28,29 All major human lymphoid- and myeloid-cell compartments, including B cells, αβ T cells, γδ T cells, natural killer (NK) cells, monocytes/macrophages, and conventional and plasmacytoid dendritic cells are observed in such HIS (BALB-Rag/γ) mice. Development of human T cells in this system critically depends on the age of the mice at the time of reconstitution, and using newborn animals ensures an efficient engraftment of the mouse thymic rudiments by human progenitors.28,29 Overall, HIS (BALB-Rag/γ) mice exhibit efficient reconstitution by human hematopoietic lineages, and this enables the in vivo study of development and function of the human immune system.
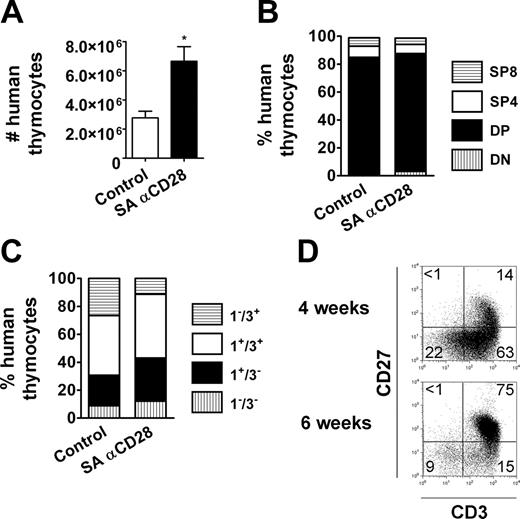
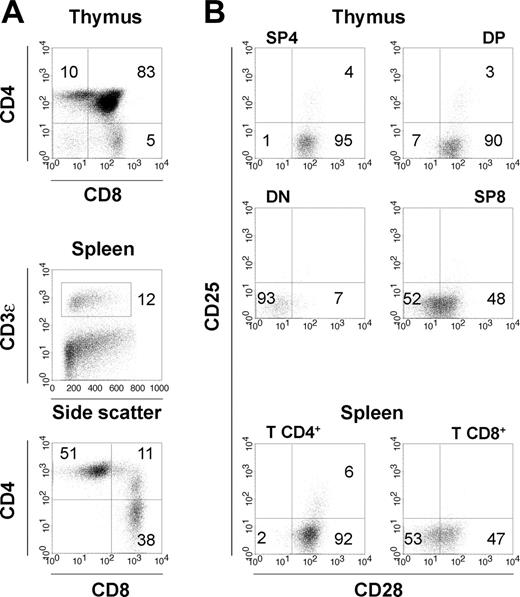
Eight to 12 weeks after injection of 0.5 to 1 × 106 CD34+ progenitors in Rag2-/-γc-/- newborn mice, we typically recovered 1 to 10 × 106 human thymocytes and 2 to 10 × 106 human splenocytes, among which 5% to 15% were T cells. Both CD4+ and CD8+ human αβ T cells developed in the murine thymus and colonized the peripheral organs of the reconstituted animals (Figure 1A), with seeding starting around the age of 5 weeks. A population of CD4+CD25+ T cells was observed both in the thymus and in the periphery (Figure 1B). Furthermore, the costimulatory molecule CD28 was expressed on the vast majority of thymocytes, most CD4+ T cells, and on half of CD8+ T cells in peripheral lymphoid organs of HIS (BALB-Rag/γ) mice (Figure 1B), similar to what is observed with peripheral-blood T cells in young human adults.34 Only 1% to 3% of all thymocytes were CD28-. It was therefore possible to study the impact of a CD28-targeting therapy on human T cells in this in vivo model.
T-cell development in HIS (BALB-Rag/γ) mice. (A) FACS profiles gated on human CD45+ cells showing T-cell development in the thymus (top) and established T-cell pool in the periphery of 8- to 12-week-old HIS (BALB-Rag/γ) mice (middle). Distribution of human T cells according to CD4 and CD8 expression is also shown for spleen CD45+CD3+ T cells (bottom). (B) Surface expression of the costimulatory molecule CD28 and the IL-2Rα/CD25 in the DN (CD4-CD8-), DP (CD4+CD8+), SP4 (CD4+CD8-CD3+), SP8 (CD4-CD8+CD3+) thymocytes populations, and mature T CD4+ and T CD8+ of the spleen. Percentages corresponding to quadrant areas or regions are indicated inside each plot.
T-cell development in HIS (BALB-Rag/γ) mice. (A) FACS profiles gated on human CD45+ cells showing T-cell development in the thymus (top) and established T-cell pool in the periphery of 8- to 12-week-old HIS (BALB-Rag/γ) mice (middle). Distribution of human T cells according to CD4 and CD8 expression is also shown for spleen CD45+CD3+ T cells (bottom). (B) Surface expression of the costimulatory molecule CD28 and the IL-2Rα/CD25 in the DN (CD4-CD8-), DP (CD4+CD8+), SP4 (CD4+CD8-CD3+), SP8 (CD4-CD8+CD3+) thymocytes populations, and mature T CD4+ and T CD8+ of the spleen. Percentages corresponding to quadrant areas or regions are indicated inside each plot.
Accumulation and accelerated differentiation of human thymocytes after treatment
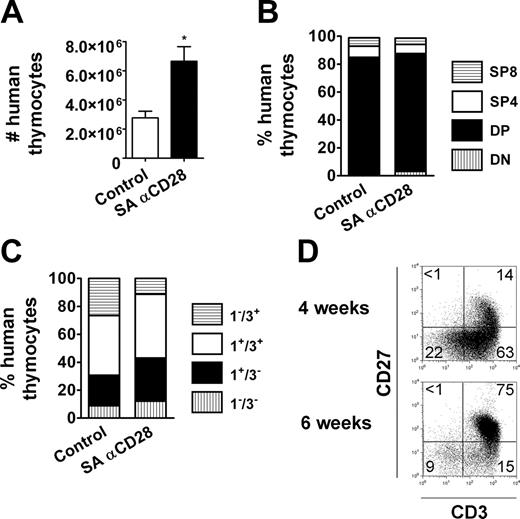
By using the HIS (BALB-Rag/γ) mouse model, we assessed the effect of SA anti-CD28 mAb therapy on human T cells in vivo. The animals were given a single injection with 0.3 mg to 0.03 mg of SA anti-CD28 mAb intraperitoneally, and control animals always received the same amount of IgG1 isotype control antibody. Three days after treatment with 0.3 mg SA anti-CD28 mAb, a substantial increase of thymus cellularity was observed, which was even more pronounced at day 6, with human thymocytes numbers being 7-fold higher in treated animals compared with control animals (Figure 2A). Using lower doses of mAb strongly reduced the effect on thymus size, with no detectable effect with 0.03 mg (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). The effect on cell numbers was accompanied by an increase in average cell size of the human thymocytes (Figure 2A) and signs of overt activation and/or differentiation, as assessed by CD25/IL-2Rα, GITR/TNFRSF18, or CD45RO expression (Figure 2B).
We analyzed the relative distribution of human thymocytes among the different thymic subpopulations, based on expression of CD4/CD8 coreceptors and maturation surface markers. Single-positive CD4+ (SP4) and CD8+ (SP8) thymocytes were the major contributors to the global increase in total cell numbers, with 18-fold and 6-fold increases at day 6, respectively (Figure 2A,C). In comparison, absolute cell numbers of the double-negative (DN) CD4-CD8- and double-positive (DP) CD4+CD8+ compartments were increased only 2-fold. The treatment was already effective as early as 3 days after injection and reached its optimum at day 6. As a consequence, more than 80% of human thymocytes were SP4 or SP8 at day 6 (Figure 2C). In addition, more than 90% of the human thymocytes exhibited a mature CD3+CD1a- phenotype at day 6 after treatment (Figure 2D), and even DP thymocyte maturation was strongly enhanced (78% of DP cells were CD3+CD1a- vs 3.6% in control animals). The absolute cell numbers of their immediate DP CD3-CD1a+ and CD3+CD1a+ progenitors were similar to controls at day 6, and reduced by 25-fold and 15-fold, respectively, at day 9 (not shown). The effect on thymus was transient, since the quantitative and qualitative composition of thymic subpopulations did not show any difference between treated and untreated animals at later time points (1-2 months) (not shown).
Overall, our observations indicate that the SA anti-CD28 treatment induces proliferation of mature SP cells and postpositive selection DP thymocytes in adult HIS (BALB-Rag/γ) mice, resulting in a transient skewing of human thymocytes to a more mature stage.
Effect of treatment on human thymocytes of young HIS (BALB-Rag/γ) mice
The previous results showed an accumulation of mature thymocytes in the thymus 6 days after SA anti-CD28 treatment of adult HIS (BALB-Rag/γ) mice, which could be due to accelerated differentiation of immature thymocytes to mature SP cells, and/or increased proliferation of already mature T cells after treatment. In order to discriminate between these 2 possibilities, we evaluated the impact of the treatment on 4-week-old reconstituted HIS (BALB-Rag/γ) mice (ie, when the thymus is mostly composed by immature thymocytes and the peripheral lymphoid organs are not yet significantly populated by human lymphocytes).
The effect on the thymus was much less pronounced in SA anti-CD28 treated 4-week-old animals, showing limited increase of thymic cellularity compared with control animals (Figure 3A). The human thymocytes were not skewed to mature single-positive T cells, although more activated CD25+ single-positive T cells could be observed in some animals (not shown). Furthermore, subset distribution based on the CD4/CD8 coreceptors or CD3/CD1a surface markers was similar between groups (Figure 3B-C), in contrast to what we observed in 8- to 12-week-old HIS (BALB-Rag/γ) animals (Figure 2C-D). In order to better understand the reason of the treatment ineffectiveness, we examined more carefully the composition of the thymus in HIS (BALB-Rag/γ) mice 4 weeks after reconstitution with human progenitors. As expected, the thymus contained at this age lower numbers of CD3+CD27+ cells (Figure 3D) (ie, positively selected thymocytes).35 This observation may explain why the thymocytes of 4-week-old HIS (BALB-Rag/γ) mice only expanded moderately after SA anti-CD28 treatment, despite the fact that CD28 was expressed on almost all thymocytes, similar to that in adult animals (Figure 1B). Experiments with HIS (BALB-Rag/γ) mice 5 weeks after reconstitution, which contain more CD3+CD27+ cells in the thymus, gave intermediate results between the situation in young (4-week-old) and adult (8- to 12-week-old) mice (not shown).
In summary, SA anti-CD28 is less effective in a situation where the thymus is immature (ie, mostly devoid of CD3+CD27+ thymocytes), indicating that SA anti-CD28 cannot bypass positive selection of human thymocytes. Thus, proliferation of already selected mature thymocytes was the major contributor to cell increase in adult HIS (BALB-Rag/γ) mice.
SA anti-CD28 treatment induces accumulation of mature thymocytes in HIS (BALB-Rag/γ) mice. (A) Number of human CD45+ thymocytes at 3, 6, and 9 days after SA anti-CD28 mAb (mean ± SEM; □ indicates control animals; ▪, treated animals; *P < .05; **P < .01). The effect on size (forward scatter [FSC]) and granularity (side scatter [SSC]) at day 6 is shown in overlay histograms, comparing thymocytes from IgG1 control (thin line) and SA anti-CD28 treated (filled histogram) mice. (B) Surface expression of CD25, GITR, and CD45RO was compared between control (thin line) and treated HIS (BALB-Rag/γ) mice (filled histogram) in the DP, SP4, and SP8 thymocyte subsets. Values in each overlay histogram indicate the percentage of positive cells in control (top) versus treated mice (bottom). (C) The relative distribution of thymocytes between the DN (▥), DP (▪), SP4 (□) and SP8 (▤) was calculated at days 3, 6, and 9 in control and treated mice. Representative FACS profiles of CD4 and CD8 expression obtained at day 6 are shown for control (top) and treated mice (bottom). (D) Similarly, the relative distribution of thymocytes according to CD1a and CD3 expression is shown, from immature CD1a-CD3-(▥), CD1a+CD3-(▪), and CD1a+CD3+ (□) to mature CD1a-CD3+ T cells (▤). Numeric results correspond to the mean values obtained from 4 mice, and are representative of 1 experiment out of 3.
SA anti-CD28 treatment induces accumulation of mature thymocytes in HIS (BALB-Rag/γ) mice. (A) Number of human CD45+ thymocytes at 3, 6, and 9 days after SA anti-CD28 mAb (mean ± SEM; □ indicates control animals; ▪, treated animals; *P < .05; **P < .01). The effect on size (forward scatter [FSC]) and granularity (side scatter [SSC]) at day 6 is shown in overlay histograms, comparing thymocytes from IgG1 control (thin line) and SA anti-CD28 treated (filled histogram) mice. (B) Surface expression of CD25, GITR, and CD45RO was compared between control (thin line) and treated HIS (BALB-Rag/γ) mice (filled histogram) in the DP, SP4, and SP8 thymocyte subsets. Values in each overlay histogram indicate the percentage of positive cells in control (top) versus treated mice (bottom). (C) The relative distribution of thymocytes between the DN (▥), DP (▪), SP4 (□) and SP8 (▤) was calculated at days 3, 6, and 9 in control and treated mice. Representative FACS profiles of CD4 and CD8 expression obtained at day 6 are shown for control (top) and treated mice (bottom). (D) Similarly, the relative distribution of thymocytes according to CD1a and CD3 expression is shown, from immature CD1a-CD3-(▥), CD1a+CD3-(▪), and CD1a+CD3+ (□) to mature CD1a-CD3+ T cells (▤). Numeric results correspond to the mean values obtained from 4 mice, and are representative of 1 experiment out of 3.
SA anti-CD28 treatment in young HIS (BALB-Rag/γ) mice. (A) The number of human CD45+ thymocytes is shown at day 6 (mean ± SEM; □ indicates control animals; ▪, treated animals; *P < .05). The relative distribution of thymocytes at day 6, according to CD4/CD8 expression (B) and CD3/CD1a expression (C), is shown as previously described in Figure 2. Numeric results correspond to the mean values obtained from 3 to 4 mice, and are representative of 1 experiment out of 2. (D) Expression of CD3 and CD27 on total thymocytes in HIS (BALB-Rag/γ) mice 4 weeks (top) and 6 weeks (bottom) after reconstitution. Proportions of CD3+CD27+ cells (mean ± SEM) calculated from 7 individual mice are as follows: 17.9% ± 5.0% at 4 weeks; 73.9% ± 8.0% at 6 weeks.
SA anti-CD28 treatment in young HIS (BALB-Rag/γ) mice. (A) The number of human CD45+ thymocytes is shown at day 6 (mean ± SEM; □ indicates control animals; ▪, treated animals; *P < .05). The relative distribution of thymocytes at day 6, according to CD4/CD8 expression (B) and CD3/CD1a expression (C), is shown as previously described in Figure 2. Numeric results correspond to the mean values obtained from 3 to 4 mice, and are representative of 1 experiment out of 2. (D) Expression of CD3 and CD27 on total thymocytes in HIS (BALB-Rag/γ) mice 4 weeks (top) and 6 weeks (bottom) after reconstitution. Proportions of CD3+CD27+ cells (mean ± SEM) calculated from 7 individual mice are as follows: 17.9% ± 5.0% at 4 weeks; 73.9% ± 8.0% at 6 weeks.
Increased thymic export of human T cells after SA anti-CD28 treatment
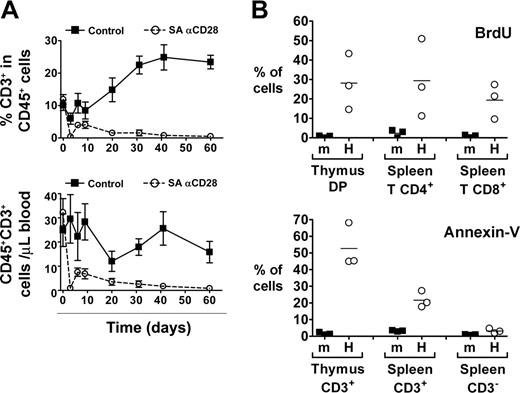
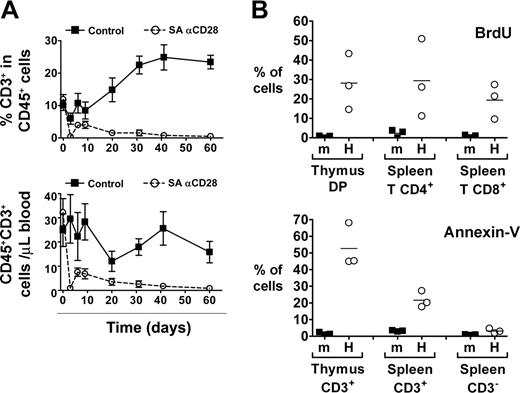
We next evaluated the impact of the SA anti-CD28 treatment on human peripheral T cells of 8- to 12-week-old HIS (BALB-Rag/γ) mice. While the frequency of T cells was constantly increasing in control animals until it reached 25% of human cells in blood, the percentage of T cells in the treated animals dropped to less than 1% after 2 months (Figure 4A). This drop was already evident 3 days after treatment and affected both CD4+ and CD8+ T cell subsets. Analysis of absolute human T-cell numbers showed identical results, and similar observations were obtained in other peripheral lymphoid organs (eg, spleen, liver, and bone marrow [not shown]). No effect of the treatment could be observed on other hematopoietic derived subsets like CD19+ B lymphocytes, CD56+ NK cells, CD14+ monocytes, or CD123+BDCA2+ plasmacytoid dendritic cells.
We consistently observed a partial recovery in peripheral T-cell numbers between day 3 (virtually no human T cells in the blood) and day 6 (around 5% T cells within human CD45+ cells) (Figure 4A), in correlation with the peak of the effect in the thymus (Figure 2). This observation suggests that thymic output was increased in treated animals between days 3 and 6. SA anti-CD28 treatment of young HIS (BALB-Rag/γ) mice did not induce any significant accumulation of peripheral T cells at day 6 in any of the performed experiments (not shown), despite the fact that thymus size was increased (Figure 3).
Since peripheral T cells were drastically depleted from lymphoid organs only 3 days after treatment, we hypothesized that human T cells found in the periphery of HIS (BALB-Rag/γ) mice could be particularly sensitive to stimuli. In absence of any treatment, we analyzed the lifespan and viability of splenic T cells by BrdU incorporation and Annexin-V staining (Figure 4B). After 1 day of BrdU treatment, 10% to 50% of human thymocytes and peripheral human T cells in HIS (BALB-Rag/γ) mice were already positive for BrdU incorporation, compared with less than 5% in wild-type BALB/c mice (Figure 4B). Furthermore, a large proportion of T cells, but not CD3- cells (mostly B cells) were also positive for Annexin-V staining. Altogether, these results indicate that human T cells in HIS (BALB-Rag/γ) mice have a high turnover and are prone to undergoing apoptosis.
SA anti-CD28 treatment induces increased human thymic output despite T-cell depletion and high T-cell turnover in the periphery of HIS (BALB-Rag/γ) mice. (A) The percentage of CD3+ T cells among CD45+ cells (top) and the absolute number of human T cells (bottom) was monitored in the blood of IgG1 control (▪) and SA anti-CD28-treated mice (○) over a 2-month period (mean ± SEM). Representative results from 1 experiment out of 3 are shown. (B) Control wild-type BALB/c mice (m) and HIS (BALB-Rag/γ) mice (H) were treated for 1 day with BrdU and incorporation by cells of the indicated subsets was analyzed (top panel). Proportion of Annexin-V+ cells was also determined in the indicated populations (bottom panel). Representative results from 1 experiment out of 2 are shown. Horizontal bars indicate mean values.
SA anti-CD28 treatment induces increased human thymic output despite T-cell depletion and high T-cell turnover in the periphery of HIS (BALB-Rag/γ) mice. (A) The percentage of CD3+ T cells among CD45+ cells (top) and the absolute number of human T cells (bottom) was monitored in the blood of IgG1 control (▪) and SA anti-CD28-treated mice (○) over a 2-month period (mean ± SEM). Representative results from 1 experiment out of 3 are shown. (B) Control wild-type BALB/c mice (m) and HIS (BALB-Rag/γ) mice (H) were treated for 1 day with BrdU and incorporation by cells of the indicated subsets was analyzed (top panel). Proportion of Annexin-V+ cells was also determined in the indicated populations (bottom panel). Representative results from 1 experiment out of 2 are shown. Horizontal bars indicate mean values.
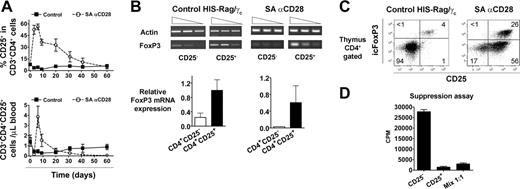
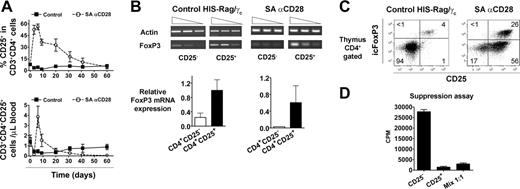
Peripheral accumulation of human suppressive CD4+CD25+icFoxP3+T cells in treated HIS (BALB-Rag/γ) mice. (A) The percentage of CD25+ cells among CD3+CD4+ T cells (top) and their absolute number (bottom) was monitored in the blood of IgG1 control (▪) and SA anti-CD28-treated mice (○) over a 2-month period (mean ± SEM). Each point shows the mean value obtained from 4 to 9 mice, and this experiment is a representative out of a total of 3. (B) The expression of FOXP3 mRNA was analyzed at day 6 by RT-PCR in sorted human CD4+CD25+ thymocytes from both control (left) and treated (right) animals. The corresponding numeric results (FoxP3/actin ratio) are shown in the bottom graphs. Representative results from 1 experiment out of 3 are shown, and similar results were obtained from spleens. (C) Thymocytes of control (left) and treated (right) animals were stained for intracellular FoxP3 (icFoxP3) expression, and results show representative staining in gated CD4+ cells. (D) CD4+CD25high and CD4+CD25-thymocytes were sorted from 6-day-treated HIS (BALB-Rag/γ) mice and cultured for suppression assay, as described in “Materials and methods.” Values are representative of 3 independent experiments.
Peripheral accumulation of human suppressive CD4+CD25+icFoxP3+T cells in treated HIS (BALB-Rag/γ) mice. (A) The percentage of CD25+ cells among CD3+CD4+ T cells (top) and their absolute number (bottom) was monitored in the blood of IgG1 control (▪) and SA anti-CD28-treated mice (○) over a 2-month period (mean ± SEM). Each point shows the mean value obtained from 4 to 9 mice, and this experiment is a representative out of a total of 3. (B) The expression of FOXP3 mRNA was analyzed at day 6 by RT-PCR in sorted human CD4+CD25+ thymocytes from both control (left) and treated (right) animals. The corresponding numeric results (FoxP3/actin ratio) are shown in the bottom graphs. Representative results from 1 experiment out of 3 are shown, and similar results were obtained from spleens. (C) Thymocytes of control (left) and treated (right) animals were stained for intracellular FoxP3 (icFoxP3) expression, and results show representative staining in gated CD4+ cells. (D) CD4+CD25high and CD4+CD25-thymocytes were sorted from 6-day-treated HIS (BALB-Rag/γ) mice and cultured for suppression assay, as described in “Materials and methods.” Values are representative of 3 independent experiments.
Transient accumulation of peripheral human CD4+CD25+FoxP3+ T cells after treatment
Since accumulation of “regulatory” CD4+CD25+ T cells was reported after SA anti-CD28 treatment in rats,27 the kinetics of this subset were analyzed (Figure 5A). Treatment of HIS (BALB-Rag/γ) mice induced a strong, but transient, increase of the CD4+CD25+ T-cell subset, and frequency of CD25+ cells reached more than 50% of CD4+ T cells after 6 days, whereas control values fluctuated around 2% to 8% in the analyzed time frame. Consequently, absolute numbers of CD4+CD25+ T cells were around 10-fold higher in treated animals at day 6, but these cells disappeared in less than 2 weeks (Figure 5A). Sorted CD4+CD25- and CD4+CD25+ T cells from both control and 6-day-treated SA anti-CD28 HIS (BALB-Rag/γ) mice were analyzed for the expression of FOXP336 by reverse transcription (RT)-PCR (Figure 5B). FOXP3 transcription in both thymus and spleen was higher in the CD25+ subset.
To more precisely enumerate the CD4+CD25+FoxP3+ T-cell subset, we performed an intracellular flow cytometric analysis by using a FoxP3-specific mAb. As already shown (Figure 2), treatment with SA anti-CD28 induced the accumulation of mature SP CD4+CD25+ T cells in the thymus, and 30% to 40% of them expressed the FoxP3 transcription factor (Figure 5C). Although the majority of CD4+ T cells were part of the activated subset (CD25+icFoxP3-), the frequency of CD25+icFoxP3+ cells was strongly increased (5- to 6-fold) after treatment. Because the absolute number of SP CD4+ cells was 15- to 20-fold higher in treated HIS (BALB-Rag/γ) mice, the total amount of CD4+CD25+icFoxP3+ T cells was drastically (approximately 100-fold) increased in the thymus at day 6 after SA anti-CD28 injection. The suppressive capacity of these CD4+CD25high thymocytes (enriched for icFoxP3+ cells) was assessed in vitro on the proliferation of their CD4+CD25- counterparts (Figure 5D). Expectedly, they exerted a strong suppressive effect, demonstrating that SA anti-CD28 treatment induces the development of human regulatory CD4+CD25+FoxP3+ T cells in the thymus of HIS (BALB-Rag/γ) mice.
Discussion
In this article, we assessed the effect of SA anti-CD28 treatment in vivo on human T cells, using the recently designed HIS (BALB-Rag/γ) mouse model. This model provides a valuable alternative for the in vivo study of development and function of the human immune system, and its therapeutic modulation.30,37 Three major effects were induced on T-cell lineage by the treatment: (1) the transient accumulation of mature thymocytes; (2) long-term depletion of peripheral T cells; and (3) the transient accumulation of suppressive CD4+CD25+FoxP3+ T cells.
At the peak of the effect (day 6), thymocyte numbers were increased up to 7-fold in SA anti-CD28-treated adult HIS (BALB-Rag/γ) mice, in a dose-dependent manner (Figures 2A, S1). The increased thymus cellularity was accompanied by skewing of the thymocytes to a more mature phenotype (SP4/8, CD1a-CD3+) (Figure 2C-D). These effects were due to increased proliferation of mature thymocytes, and our results argue against accelerated differentiation of DP thymocytes after treatment. The effect of the treatment was much less on human thymocytes at 4 weeks of age, when production of fully mature T cells is not yet achieved, only inducing a limited increase of cellularity without phenotypic skewing (Figure 3). Therefore, positively selected mature SP thymocytes are the major target of SA anti-CD28 mAb in the thymus and SA anti-CD28 treatment does not bypass T-cell-positive selection. As far as thymopoiesis recovery after BM transplantation is concerned, it can therefore be speculated that the use of SA anti-CD28 would potentially be of interest when mature T cells have already accumulated in the thymus.
Because thymocyte accumulation is observed to some extent, SA anti-CD28 treatment in 4-week-old HIS (BALB-Rag/γ) mice is not inert, although not fully efficient. One explanation for these results is that only 6- to 8-week-old but not 4-week-old mice contain many more mature SP T cells. Indeed, we found that the thymus of 4-week-old HIS (BALB-Rag/γ) mice contain only 4.3% ± 0.8% CD4+CD3+CD1a- SP thymocytes versus 35.9% ± 7.5% of these cells at week 6. Over this 2-week period of time, their absolute numbers increased up to 16-fold (from 0.19 × 106 ± 0.03 × 106 at 6 weeks of age to 2.99 × 106 ± 0.31 × 106 at 6 weeks of age). These latter cells may be the main target for SA anti-CD28 resulting in an extensive proliferation. Nonetheless, the thymus of the 4-week-old mice increased 2- to 3-fold in cell numbers following treatment with SA anti-CD28, while the distribution of the various subsets was not significantly affected. These results suggest that CD28, which is expressed on very early CD34+ thymic precursors, on CD4 immature single-positive cells, and on DP cells that have passed the β-selection checkpoint (S. J. Ligthart, Y. Yasuda, H.S., and B. Blom, manuscript submitted), can transmit proliferation-inducing signals after SA anti-CD28 cross-linking. It was already shown that the T-cell proliferative effect induced by SA anti-CD28 requires the presence of TCR complex and its direct downstream signaling effectors (reviewed in Hunig and Dennehy38 ). It is hypothesized that the TCR and CD28 signaling pathways merge at the level of SLP-76 adaptor protein, which is a direct target of downstream TCR kinase ZAP-70 and downstream CD28 guanine nucleotide exchange factor Vav.38 The limited SA anti-CD28 effects seen in 4-week-old HIS (BALB-Rag/γ) animals may therefore reflect activation of CD28-specific TCR-independent pathways in immature thymocytes.
In contrast to what was previously reported in rats,25,26 the treatment induced a rapid and specific depletion of human peripheral T cells. Mature T lymphocytes were almost undetectable at day 3 after treatment (Figure 4). Between day 3 and day 6, a partial recovery in human peripheral T-cell numbers was observed in SA anti-CD28 treated-HIS (BALB-Rag/γ) mice, correlating with the effect seen in the thymus. This observation argues for a transient increase of thymic output due to the effect of the treatment on thymocytes, which in turn leads to a transient accumulation of mature T cells in the periphery. In a previous study, Lewis rats were lethally irradiated and reconstituted with syngeneic bone marrow (CD45a) and CD45b congenic mature T cells, with or without SA anti-CD28 treatment.26 In this experimental setting, the rate of de novo CD45a+ T-cell production was evaluated in the blood and was found unmodified after SA anti-CD28 treatment, or moderately increased at 2 weeks, but detailed analysis of the rat thymuses was not performed.26 In a situation of optimal thymic output and/or recovery (eg, in the previously mentioned reconstituted rats), SA anti-CD28 treatment may not be able to further increase thymic production. In contrast, a beneficial effect may be revealed in suboptimal conditions (eg, in HIS (BALB-Rag/γ) mice, which show to some extent characteristics of chronic T-cell lymphopenia and limited thymic output).
Despite a T-cell increase in SA anti-CD28-treated animals between days 3 and 6, the T-cell pool did not recover completely when monitored over a 2-month period (Figure 4). Even a low dose of 5.11A1 SA anti-CD28 mAb (0.01 mg) induced a long-lasting human T-cell depletion (not shown), with no impact on other human immune cell subsets in the periphery. It has to be pointed out that T-cell depletion was never observed in treated rats, Rhesus or Cynomolgus monkeys, or huCD28 transgenic mice treated with the same 5.11A1 mAb (T.H., unpublished observations, 2004-2005). Several hypotheses may explain this unexpected effect in HIS (BALB-Rag/γ) mice. It is possible that the anti-human CD28 mouse IgG1 used in the experiments is involved in complement-induced lysis and/or antibody-dependent cell-mediated cytotoxicity of targeted T cells,39 since some murine hematopoiesis-derived cells are still present in the HIS (BALB-Rag/γ) mice.28,29 Experiments using a humanized version of the 5.11A1 clone could be useful to exclude or confirm this hypothesis, but the absence of depletion in the previously mentioned huCD28 transgenic mice makes it unlikely. Alternatively, T-cell depletion in the periphery could be due to a defective T-cell survival in HIS (BALB-Rag/γ) mice. The large proportions of BrdU+ cycling T cells (10%-50% depending on the subset) and apoptotic Annexin-V+ T cells (around 25% in the spleen) observed in unmanipulated HIS (BALB-Rag/γ) mice support this idea (Figure 4B). It has been described that T cells placed in a lymphopenic environment undergo “homeostatic” lymphopenia-driven proliferation, due to sudden availability of usually limiting resources (such as IL-7 or self-peptide/MHC molecule complexes on antigen-presenting cells) and/or space.4,5 A short life-span of T cells in HIS (BALB-Rag/γ) mice may also be linked to mismatch between human and mouse molecules involved in T-cell survival (eg, interactions between TCR and MHC molecules, interleukins and their receptors, or costimulatory molecules and their ligands).4,5 In such conditions, surviving T cells could be extremely sensitive to any stimulus, and easily enter activation-induced cell death, despite the fact that SA anti-CD28 activated rat T cells were shown to up-regulate the expression of the survival factor Bcl-xL.40
Rapid accumulation of CD4+CD25+ T cells with suppressive capacity was transiently observed after SA anti-CD28 treatment in rats.27 Despite T-cell depletion, we also observed a strong but transient accumulation of CD4+CD25+ T cells in both central (approximately 100-fold in thymus) and secondary lymphoid organs (approximately 10-fold in blood) of treated HIS (BALB-Rag/γ) mice, peaking at day 6 (Figure 5). The resulting CD4+CD25+ T cells were enriched for the expression of FoxP3, both at mRNA and protein levels. The expression of the Forkhead family transcription factor FoxP3 was described as specifically driving the development of regulatory CD4+CD25+ T cells.36,41,42 Still, its expression—perhaps another splice variant—has also been reported in activated human T-cell clones devoid of any suppressive activity.43 Here, we report that CD4+CD25hi T cells (enriched for FoxP3+ cells) isolated from the thymus of treated HIS (BALB-Rag/γ) mice were suppressive in vitro.
Our observations that SA anti-CD28 treatment increased the number of FoxP3+ cells in the thymus are in accordance with the recent report demonstrating increased FOXP3 expression in immature (CD4+CD8+) murine thymocytes after concomitant TCR and CD28 ligation.44 Similarly, several studies have demonstrated a role of CD28/CTLA-4 triggering by B7.1/B7.2 in the development and/or maintenance of regulatory T (Treg) cells.45-48 Since Treg cells did not accumulate in the thymus of 4-week-old treated animals, our results indicate that SA anti-CD28-induced accumulation of thymic Treg cells requires full maturation of CD4+ T cells. Thus, SA anti-CD28 may induce the expansion of already functional Treg cells, rather than promoting their development from DP thymocytes. Interestingly, SA anti-CD28 treatment resulted also in expansion of a CD4+CD25+FoxP3- T-cell population (Figure 5C), indicating that CD28-mediated activation of CD4+ T cells per se does not induce FOXP3 expression, hence supporting the notion that FoxP3 is a selective marker of activated Treg cells in vivo.
T-cell lymphopenia is associated with several human autoimmune diseases, including insulin-dependent diabetes mellitus, rheumatoid arthritis, and lupus.9 Similar observations have been obtained in rodent models, and it was recently shown in the diabetes-prone nonobese diabetic (NOD) mouse model that chronic lymphopenia leads to activation of effector T cells undergoing homeostatic proliferation, which will eventually results in tissue damage.49,50 Using SA anti-CD28 mAb in rats was efficient enough to block the induction of experimental autoimmune neuritis51 and experimental autoimmune encephalomyelitis.52 It is likely that such a beneficial effect of SA anti-CD28 treatment against T-cell-mediated autoimmune diseases resides in the preferential expansion of CD4+CD25+FoxP3+ T cells with regulatory capacities.24,51-53 Here, we demonstrated that SA anti-CD28 treatment in adult HIS (BALB-Rag/γ) mice leads to in vivo accumulation of human regulatory T cells in the thymus, with a peak effect around day 6 after onset of treatment. From a translational perspective, such effect on thymopoiesis and Treg accumulation is of major interest as far as the de novo produced functional regulatory T cells can exert beneficial regulatory functions on potential autoimmune syndromes. More detailed studies will be needed to prove that in vivo SA anti-CD28-induced development of human regulatory T cells can be beneficial for the treatment of experimentally induced autoimmune pathology.
Supported by a research grant from Landsteiner Blood Transfusion Research Foundation (LSBR no. 2003-1365).
T.H. is cofounder and Chief Scientific Officer of TeGenero Immuno Therapeutics AG. TeGenero develops CD28 superagonists for clinical use. The authors have no other potential conflicting financial interests.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2006-01-0190.
We thank Christel Uittenbogaart, Björn Clausen, and Mireille Centlivre for helpful comments, discussions, and review of the manuscript. We acknowledge the Bloemenhove Clinic (Heemstede, The Netherlands) for providing fetal tissues. We thank Suzanne Ligthart and Wendy Dontje for fetal material preparation, Berend Hooibrink for cell sorting and maintenance of the FACS facility, and Angelique Epping and Lydia Wolterman for animal care.

![Figure 2. SA anti-CD28 treatment induces accumulation of mature thymocytes in HIS (BALB-Rag/γ) mice. (A) Number of human CD45+ thymocytes at 3, 6, and 9 days after SA anti-CD28 mAb (mean ± SEM; □ indicates control animals; ▪, treated animals; *P < .05; **P < .01). The effect on size (forward scatter [FSC]) and granularity (side scatter [SSC]) at day 6 is shown in overlay histograms, comparing thymocytes from IgG1 control (thin line) and SA anti-CD28 treated (filled histogram) mice. (B) Surface expression of CD25, GITR, and CD45RO was compared between control (thin line) and treated HIS (BALB-Rag/γ) mice (filled histogram) in the DP, SP4, and SP8 thymocyte subsets. Values in each overlay histogram indicate the percentage of positive cells in control (top) versus treated mice (bottom). (C) The relative distribution of thymocytes between the DN (▥), DP (▪), SP4 (□) and SP8 (▤) was calculated at days 3, 6, and 9 in control and treated mice. Representative FACS profiles of CD4 and CD8 expression obtained at day 6 are shown for control (top) and treated mice (bottom). (D) Similarly, the relative distribution of thymocytes according to CD1a and CD3 expression is shown, from immature CD1a-CD3-(▥), CD1a+CD3-(▪), and CD1a+CD3+ (□) to mature CD1a-CD3+ T cells (▤). Numeric results correspond to the mean values obtained from 4 mice, and are representative of 1 experiment out of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/1/10.1182_blood-2006-01-0190/2/m_zh80130697830002.jpeg?Expires=1764550563&Signature=xFNnRuqAEBDAs0ISfqtXdQl9LHnVi9KTxCjJjX4u6ag00qU5EbS2jN9cBG8kTSkOI6I798fWf3GJ7Do8sjD0J7hyie5xnr6LKYeVG~d4SizND~4-Ebb~KuOvLifskXHpX2Qudsstbqw9pahachP2xZn8gjwKfG~ib0Cp9TU0616NLxTyqW5gjz0Rm5TzX1cyku69WIRfWVJaEkWmRtz03J7Va-x5JzxrDwAWPGyKfgAMkiXKjgFtbtnXVD32WqEUzoydgK7dxjE-EIxjziWXzE7ycFl3tMIQ4JKZCpDT57CKi1SDDWHmuPyu1YakpddNtkJjcxCFbhFFr1HENeCMiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. SA anti-CD28 treatment induces accumulation of mature thymocytes in HIS (BALB-Rag/γ) mice. (A) Number of human CD45+ thymocytes at 3, 6, and 9 days after SA anti-CD28 mAb (mean ± SEM; □ indicates control animals; ▪, treated animals; *P < .05; **P < .01). The effect on size (forward scatter [FSC]) and granularity (side scatter [SSC]) at day 6 is shown in overlay histograms, comparing thymocytes from IgG1 control (thin line) and SA anti-CD28 treated (filled histogram) mice. (B) Surface expression of CD25, GITR, and CD45RO was compared between control (thin line) and treated HIS (BALB-Rag/γ) mice (filled histogram) in the DP, SP4, and SP8 thymocyte subsets. Values in each overlay histogram indicate the percentage of positive cells in control (top) versus treated mice (bottom). (C) The relative distribution of thymocytes between the DN (▥), DP (▪), SP4 (□) and SP8 (▤) was calculated at days 3, 6, and 9 in control and treated mice. Representative FACS profiles of CD4 and CD8 expression obtained at day 6 are shown for control (top) and treated mice (bottom). (D) Similarly, the relative distribution of thymocytes according to CD1a and CD3 expression is shown, from immature CD1a-CD3-(▥), CD1a+CD3-(▪), and CD1a+CD3+ (□) to mature CD1a-CD3+ T cells (▤). Numeric results correspond to the mean values obtained from 4 mice, and are representative of 1 experiment out of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/1/10.1182_blood-2006-01-0190/2/m_zh80130697830002.jpeg?Expires=1764550564&Signature=s0VSlCrP4qzgx1ZUiUyQtkk72YY-g2fbT~1tYUmpGQyAe65PxxMyfK-9wNccbvIl~avYDK9pGxdRHxu9ek0CeQTgoCjq9NspkWhmD1iQV6yfaTsowuaycTlVoOgJ0spsS29vZhmUJUHVs01p0SB9u8TRRUpFSZDcAH3Ar2lzAgM8pvzrp7LRyV1dV64VoK3QvVsxcOZdwwoD~6BeRAex0onaEGYx0btCsfz~2tyBtwcL-kltT3j8Ps6bujvvdK0~3ci7gz51MCzEmA1X0BYu~glD~GjU0pqZcCsO2ZmYsiTcXWJSpeR6~qFZVoEzvte4hhk9Um~iqRK95Db4pB~ypQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)