Shedding of adhesion molecules has been described for members of the selectin and immunoglobulin superfamilies, but integrins are not known to be shed. Here, we describe shedding of the integrin lymphocyte function–associated antigen-1 (LFA-1; CD11a/CD18) from human leukocytes during the cutaneous inflammatory response to the blistering agent cantharidin. Expression of LFA-1 was significantly diminished on blister-infiltrated neutrophils (P < .001) and monocytes (P = .02) compared with cells in peripheral blood, but expression on lymphocytes remained unchanged. A capture enzyme-linked immunosorbent assay (ELISA) indicated that LFA-1 was shed into blister fluid as a heterodimer expressing an intact headpiece with I and I-like epitopes. However, a CD11a central region epitope, G25.2, was absent and this remained expressed as a “stub” on the cell surface of blister neutrophils. Western analysis of soluble LFA-1 revealed a truncated 110-kDa CD11a chain and a minimally truncated 86-kDa CD18 chain. However, LFA-1 was shed in a ligand-binding conformation, since it expressed KIM-127 and 24 activation epitopes and bound to solid-phase ICAM-1. Shed LFA-1 was also detected in a synovial effusion by ELISA and Western analysis. We hypothesize that LFA-1 shedding may play a role in leukocyte detachment after transendothelial migration and in regulating integrin-dependent outside-in signaling.
Introduction
Leukocyte adhesion molecules are key mediators of inflammation, controlling the recruitment, subendothelial migration, and activation of leukocytes at sites of injury or infection. The adhesiveness and signaling potential of adhesion receptors are regulated at the level of receptor expression, distribution on the cell surface, and affinity modulation. Shedding of adhesion receptors has been demonstrated for the selectin and immunoglobulin superfamilies.1,2 In the case of L-selectin, shedding is not essential for initial leukocyte–endothelial-cell interactions but is important for subsequent leukocyte migration.3,4 At present, there is little evidence for shedding of integrins, although proteolysis of the common β2 subunit of the leukocyte integrin, CD18, has recently been described under the effect of shear stress.5
LFA-1 is an integrin receptor expressed exclusively by leukocytes, which is involved in each step of the leukocyte–endothelial-cell adhesion cascade (rolling, firm adhesion, transmigration, and subendothelial migration) and cognate interactions between T cells and antigen-presenting cells.6-12 Its main ligand is intercellular adhesion molecule-1 (ICAM-1), widely expressed on endothelium, subendothelial cells, and leukocytes.13 Adhesiveness of LFA-1 is tightly controlled by a combination of receptor-transduced (inside-out) signals and adhesion-strengthening signals emanating from ligand binding (outside-in) that together modify the LFA-1 conformation, precluding adhesion events from taking place in the bloodstream or at sites distant from the inflammatory target.14 In the inactive form, the LFA-1 α/β heterodimer (CD11a/CD18) has a bent conformation that renders the ligand-binding headpiece less accessible.15-16 In the active conformation, LFA-1 assumes an extended structure that exposes the ligand-binding site on the I domain of the α subunit headpiece.10,11 The extended conformation is recognized by the reporter antibodies KIM-127 and 24, which bind to epitopes exposed on the β subunit and α/β headpiece, respectively.15-18 It is presently thought that the switch between active and inactive conformers is controlled by a membrane-proximal motif on the cytoplasmic domain of the β subunit that exists in a state of competition with the intracytoplasmic region on the α subunit (promoting a low-affinity state) and the cytoskeletal protein talin (promoting a high-affinity state).19-21
LFA-1 has been proposed to play a central role in human neutrophil transmigration by enabling extravasating cells to squeeze through endothelial junctions using a ring of LFA-1:ICAM-1.22 As a consequence of this transmigration strategy, LFA-1 accumulates at the trailing edge of the transmigrated neutrophil, posing the question of how it then detaches from ICAM-1 on the basal side of the endothelium. The answer to this question remains unknown, but studies on neutrophils migrating on fibronectin using their β1 integrins demonstrate that uropod retraction occurs via a calcium- and myosin II–dependent mechanism, whereas the trailing edge on transmigrating monocytes and T cells is retracted via a ROCK/Rho A activation pathway, possibly through negative regulation of β2 integrin adhesion.23-25 Bulky glycoproteins, such as CD43, may also play a role in de-adhesion of lymphocytes from ICAM-1.26 The precise mechanisms controlling LFA-1 detachment thus remain poorly understood, except that de-adhesion is an essential requirement for neutrophil extravasation in inflammation and the generation of T-cell immune responses.27
Following extravasation, LFA-1 transduces further outside-in signals from the subendothelial environment to regulate inflammatory programs and reactive oxygen species secretion by neutrophils and monocytes.28-31 Understanding how leukocytes regulate LFA-1 expression and function during the course of an inflammatory response is therefore of critical importance. However, the difficulty in performing controlled inflammatory experiments in humans has meant that the effect of in vivo extravasation on integrin expression has never been analyzed in humans in detail. Only limited work has been performed on β1 integrin expression in vivo using a suction blister model of leukocyte transmigration.32 Most studies examining the effect of extravasation on integrin expression have either concentrated on animal models or used in vitro models of human transendothelial migration.
The cantharidin skin blister model provides an excellent tool to study leukocyte trafficking in a controlled human early inflammatory response.33 The basic technique can be adapted to examine the phenotype and activation state of extravasated cells in blister fluid.34,35 Here, we have used the cantharidin skin test to study the effect of extravasation on LFA-1, macrophage antigen-1 (Mac-1), and very late antigen-4 (VLA-4) expression on transmigrated leukocytes. This revealed an unexpected loss of LFA-1 from the cell surface of neutrophils and monocytes, but not lymphocytes, with concomitant appearance of shed LFA-1 in blister fluid by enzyme-linked immunosorbent assay (ELISA) and immunoblotting. LFA-1 was shed as an α/β heterodimer in the active conformation and bound to immobilized ICAM-1. Soluble LFA-1 was also detected in a synovial effusion from a patient with arthritis, demonstrating that LFA-1 shedding is not restricted to the blister model. This is the first description of LFA-1 shedding, which has implications for detachment and outside-in integrin signaling.
Patients, materials, and methods
Reagents
Cantharidin (Cantharone) was purchased from Dormer Laboratories (Rexdale, ON). FITC-conjugated anti-CD11a (clone 38), PE-conjugated anti-CD11b (clone ICRF44), anti–CD16-FITC, and control IgG conjugates were purchased from Serotec (Kidlington, United Kingdom). Anti-CD11a clone G25.2, anti–CD49d-RPE, and anti–CD62L-RPE were purchased from BD-Pharmingen (Oxford, United Kingdom). Anti–CD14-ECD and control IgG-ECD were purchased from Beckman Coulter (High Wycombe, United Kingdom). Biotinylated anti-CD11a (clone 38) was purchased from Biodesign International (Saco, MN). Goat polyclonal anti-CD18 C-terminus–specific antibody C-20 was purchased from Autogen Bioclear UK (Calne, United Kingdom). Hybridomas secreting anti-CD11a mAb TS1/22 and anti-CD18 mAb TS1/18 were purchased from the American Type Culture Collection (ATCC, Rockville, MD). Antibodies 6.5E and 24 were available in-house. Anti-CD11a antibody MEM48 was the gift of Dr Vaclav Horejsi (Institute of Molecular Genetics, Prague, Czech Republic). Anti-CD18 antibodies KIM-185 and KIM-127 were the gift of Dr Martyn Robinson (Celltech Chiroscience, Slough, United Kingdom). Anti-CD18 antibody 2E7 was the gift of Dr Carl Gahmberg (Department of Bioscience, University of Helsinki, Finland).
The cantharidin skin blister model
Cantharidin (0.1% solution in acetone) was applied topically to the forearm skin of 9 healthy volunteers to induce skin blister formation and leukocyte extravasation, as previously described.34 Institutional review board (IRB) approval and informed consent were obtained in accordance with the Declaration of Helsinki. Blister fluid was collected after 16 or 40 hours into siliconized microcentrifuge tubes (Sigma Aldrich, St Louis, MO) and stored on ice prior to cell counting and flow cytometric analysis. Differential cell counts were performed using Kimura stain,36 followed by counting in a hematocytometer. Blister supernatants were collected after microcentrifugation and stored at –70°C prior to ELISA and Western blot analysis.
Flow cytometric analysis
Flow cytometric analysis of blister leukocytes was performed immediately after microcentrifugation of blister samples, with all incubation and washing steps carried out in ice-cold PBS, as described.34 The following antibody pairs were analyzed in FL-1/FL-2 channels: LFA-1/Mac-1, VLA-4/CD16, and L-selectin/CD163. Flow cytometric analysis was carried out using an EPICS XL-3 cytometer (Beckman Coulter) and results were expressed as mean fluorescent intensities ± SD from n = 9 donors, or as representative histogram overlays or dot plots.
Collection of synovial fluid
Synovial fluid was collected by arthroscopy from a clinically swollen knee joint of an 18-year-old man presenting with a first episode of acute monoarthritis. IRB approval and informed consent were obtained. The patient was later diagnosed as having psoriatic arthritis. Synovial fluid cells were immediately analyzed by flow cytometry to determine integrin expression, and supernatants were stored at –70°C prior to ELISA and Western blot analysis.
Western blot analysis
Control lysates were prepared from neutrophils isolated from the 74% interface following discontinuous Percoll sedimentation of citrated blood to provide a reference standard for full-length CD11a and CD18 chains. Lysis and Western blotting were carried out as described previously.37 Blister and synovial fluid samples were initially depleted of albumin by passage twice over an albumin removal column (Vivascience, Hanover, Germany) prior to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS/PAGE) on a 12.5% gel under reducing conditions. Detection of CD11a was performed using mAb 38 at 1 μg/mL. Detection of CD18 was performed using mAb 2E7 or polyclonal anti–C-terminus antibody C-20 at 1 μg/mL.
LFA-1 ELISA
An LFA-1–specific sandwich ELISA was developed using a variety of CD11a and CD18 capture antibodies coated at 5 μg/mL in carbonate buffer (pH 9.6) onto 96-well ELISA plates (Nunc Maxisorb; Nalge-Nunc International, Rothkilde, Denmark) at room temperature overnight. After 3 washes in PBS supplemented with 0.05% Tween-20 (PBS-T), plates were blocked with 1% BSA and 5% sucrose in PBS for 1 hour at room temperature. After 3 further washes with PBS-T, blister fluid was added at 1:1 dilution in PBS supplemented with 1% BSA and incubated at room temperature for 2 hours. Captured LFA-1 was detected with biotinylated CD11a antibody (clone 38) at 1 μg/mL followed by color detection using streptavidin:HRP (Dako, Ely, United Kingdom). In some experiments, ICAM-1–Fc (5-domain) was used in the capture step; in those experiments, all reactions and washes following the addition of blister fluid were carried out at room temperature in Tris-buffered saline supplemented with 1 mM MgCl2 and 0.5 mg/mL BSA. The LFA-1 standard consisted of a CD11a-Fc/CD18-Fc heterodimeric fusion protein, containing the hinge exon and IgG1 Fc tail added at amino acid position KQM1089 on CD11a and GPN700 on CD18 (the gift of Dr Steven Ludbrook, Integrin Biology, GSK, Stevenage, United Kingdom). All analyses were carried out in duplicate wells, and results were expressed as mean soluble LFA-1 ± SEM (ng/mL).
Statistical analysis
Leukocyte receptor expression between blood versus cantharidin skin blisters was analyzed using a paired Student t test (GraphPad Prism Software, San Diego, CA). Significance was assumed at P < .05.
Results
Leukocyte infiltration into cantharidin skin blisters
Leukocytes in skin blister fluid were examined at 16 hours following topical application of cantharidin in 9 healthy individuals (Figure 1A). Total cell counts and blister fluid volumes were similar to those previously reported,33 with a median cell infiltrate of 8.1 × 105 cells per blister (range, 0.91-25.40 × 105) and a median volume of 0.50 mL (range, 0.15-1.02 mL). Differential counting with Kimura stain showed that neutrophils comprised the main leukocyte infiltrate (67.15% ± 17.63% of total cells in blisters [mean ± SD]), followed by monocytes (17.81% ± 3.04%), lymphocytes (9.55% ± 1.70%), and eosinophils (5.49% ± 0.87%). The granulocyte population in blister fluid was identified flow cytometrically from characteristic forward- and side-scatter properties (Figure 1B). Cells in the granulocyte gate were 94.6% ± 3.7% CD16+VLA-4– (mean ± SD), consistent with a neutrophil phenotype. The proportion of viable neutrophils in the granulocyte gate was determined from the proportion of cells staining CD16high (Figure 1B inset), as loss of CD16 (CD16lo) has been previously shown to accompany neutrophil apoptosis.38 Viable cells thus constituted 89.95% ± 3.96% of neutrophils in blister fluid (mean ± SD). Monocyte and lymphocyte populations were resolved within the mononuclear-cell (MNC) gate using anti-CD14 antibody in the fluorescent 3 (FL-3) channel. These gating strategies were next used to examine leukocyte integrin expression in the FL-1 and FL-2 channels in blood versus blisters.
Leukocyte infiltration into cantharidin-induced skin blisters. Duplicate skin blisters were established by topical application of cantharidin (0.1%) to the forearm of 9 healthy individuals. Blister fluid was collected at 16 hours and analyzed: (A) for fluid volume and cellularity (each point represents the mean from 2 skin blisters; horizontal line represents the population median) and (B) by flow cytometry to distinguish leukocyte subpopulations. The representative dot plot in panel B shows gating into distinct granulocyte and mononuclear-cell populations by characteristic forward- and side-scatter properties. The inset shows CD16 and VLA-4 staining in the granulocyte gate, which allows neutrophils (CD16+VLA-4–) and eosinophils (CD16–VLA-4+) to be resolved and, furthermore, allows the proportion of apoptotic neutrophils (CD16dimVLA-4–) to be determined. CD14 staining in the FL-3 channel was used to resolve cells in the mononuclear-cell (MNC) gate into monocyte (CD14+) and lymphocyte (CD14–) populations.
Leukocyte infiltration into cantharidin-induced skin blisters. Duplicate skin blisters were established by topical application of cantharidin (0.1%) to the forearm of 9 healthy individuals. Blister fluid was collected at 16 hours and analyzed: (A) for fluid volume and cellularity (each point represents the mean from 2 skin blisters; horizontal line represents the population median) and (B) by flow cytometry to distinguish leukocyte subpopulations. The representative dot plot in panel B shows gating into distinct granulocyte and mononuclear-cell populations by characteristic forward- and side-scatter properties. The inset shows CD16 and VLA-4 staining in the granulocyte gate, which allows neutrophils (CD16+VLA-4–) and eosinophils (CD16–VLA-4+) to be resolved and, furthermore, allows the proportion of apoptotic neutrophils (CD16dimVLA-4–) to be determined. CD14 staining in the FL-3 channel was used to resolve cells in the mononuclear-cell (MNC) gate into monocyte (CD14+) and lymphocyte (CD14–) populations.
Expression of leukocyte adhesion molecules in blood versus cantharidin skin blisters. Granulocytes, monocytes, and lymphocytes in blood and blister fluid, gated according to the strategy defined in Figure 1, were analyzed flow cytometrically for expression of LFA-1 (A), Mac-1 (B), VLA-4 (C), and L-selectin (D). Results are expressed as the mean ± SD mean fluorescent intensity (MFI) of staining from n = 9 individuals. NS indicates not significant.
Expression of leukocyte adhesion molecules in blood versus cantharidin skin blisters. Granulocytes, monocytes, and lymphocytes in blood and blister fluid, gated according to the strategy defined in Figure 1, were analyzed flow cytometrically for expression of LFA-1 (A), Mac-1 (B), VLA-4 (C), and L-selectin (D). Results are expressed as the mean ± SD mean fluorescent intensity (MFI) of staining from n = 9 individuals. NS indicates not significant.
Expression of leukocyte integrins in cantharidin skin blisters
Integrin expression on granulocytes, monocytes, and lymphocytes was compared between blood and blister. This showed an unexpected 72.8% drop in LFA-1 expression on granulocytes upon transmigration into blisters (P < .001) and a 34.4% drop on monocytes (P = .02, Figure 2A). Mac-1 by contrast was significantly up-regulated on both granulocytes (P < .001) and monocytes (P = .001), suggesting activation of cells during recruitment into the blisters (Figure 2B). No VLA-4 expression was detected on neutrophils, either in blood or in blister fluid (Figure 2C). VLA-4 expression on monocytes was not significantly altered between blood and blisters (Figure 2C). Lymphocytes showed no significant change in expression of any of the integrins comparing blood and blisters (Figure 2A-C). As expected, L-selectin expression was significantly lower on all blister-infiltrated leukocyte populations compared with blood (P = .002 to P = .001), consistent with shedding during their recruitment to the inflamed tissue (Figure 2D).1
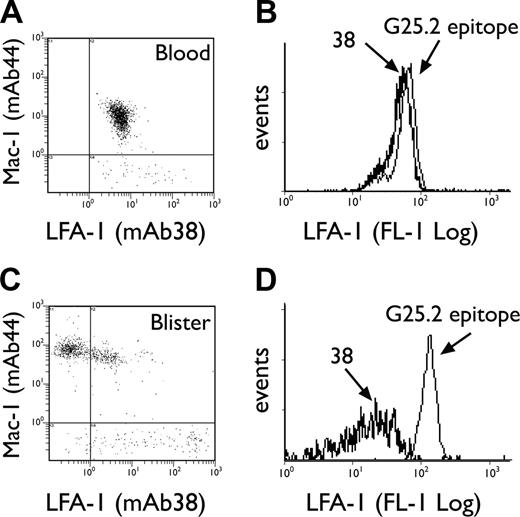
Expression of CD11a epitopes on neutrophils
The LFA-1/Mac-1 dot plots depicted in Figure 3A and C emphasize the apparent selectivity of LFA-1 loss compared with other integrins, since Mac-1 expression is simultaneously increased on the same granulocyte population in blisters. Loss of CD11a epitopes was next investigated by comparing staining with an I-domain–specific antibody, mAb 38, versus an antibody recognizing a central region epitope of CD11a, G25.2.39,40 Both epitopes were equally expressed by granulocytes in whole blood (Figure 3B); however, the 38 epitope was selectively lost upon transmigration into blisters (Figure 3D). G25.2 remained expressed as a “stub” epitope on the cell surface (Figure 3D). The loss of the 38 epitope on blister-infiltrated granulocytes was partially reversible when the cantharidin skin test was extended to 40 hours. Thus, parallel re-expression of LFA-1 38 epitope (P = .002) and L-selectin (P = .001) was noted on viable granulocytes at 40 hours following blister formation, with a concomitant increase in the density of G25.2 epitope over this time (Figure 4A-B).
Expression of granulocyte CD11a epitopes in blood versus skin blisters. Representative LFA-1/Mac-1 dot plots for neutrophils in blood (A) and 16-hour blister fluid (C), using mAbs 38:FITC and ICRF44:PE, respectively. Representative histogram overlays for CD11a epitopes 38 and G25.2 expressed by neutrophils in blood (B) and 16-hour blisters (D).
Expression of granulocyte CD11a epitopes in blood versus skin blisters. Representative LFA-1/Mac-1 dot plots for neutrophils in blood (A) and 16-hour blister fluid (C), using mAbs 38:FITC and ICRF44:PE, respectively. Representative histogram overlays for CD11a epitopes 38 and G25.2 expressed by neutrophils in blood (B) and 16-hour blisters (D).
Shedding of CD11a and CD18 subunits into blister fluid
The possibility that truncated LFA-1 subunits might be shed during leukocyte recruitment in inflammation was examined by Western blotting of blister fluid. Synovial fluid collected from a patient with psoriatic arthritis was also analyzed. The CD11a subunit was detected as a truncated protein of apparent electrophoretic mobility 110 kDa in cell-free blister fluid and patient synovial fluid, compared with reference full-length (180 kDa) in neutrophil lysates (Figure 5A). CD18 was detected as a minimally smaller subunit than the reference 90-kDa chain in blister and patient synovial fluid, with an apparent electrophoretic mobility of 86 kDa (Figure 5A-B). CD18 shed into blister fluid did not react with the C-terminal–specific blotting antibody C-20 (Figure 5C).
LFA-1 is shed as a ligand-binding heterodimer
An LFA-1–specific capture ELISA was next developed to confirm the presence of shed soluble LFA-1 (sLFA-1) in blister and synovial fluid. These experiments used 2 nonoverlapping CD11a I-domain antibodies, TS1/22 and 38,39,41 for the capture and detection steps, respectively. The TS1/22 capture ELISA demonstrated that CD11a was present in blister fluid at a concentration of 5965.0 ± 4.05 ng/mL (mean ± SEM; Figure 6A). In contrast, mAb G25.2 did not capture CD11a protein, consistent with the observation that this epitope was retained at the cell surface. The possibility that sLFA-1 may have been expressed on ectosomes released from the cell membrane was ruled out by centrifugation of blister samples at 14 000g,42 which showed no significant depletion of detectable sLFA-1 in the ELISA assay (data not shown). The patient synovial effusion contained sLFA-1 with similar antigenic properties as in blister fluid, with capture by mAb TS1/22 (3392.8 ± 101.4 ng/mL) but not G25.2.
Expression of neutrophil adhesion molecules over time in the cantharidin skin test. CD11a epitopes (A) and L-selectin (B) expressed on neutrophils in whole blood, 16-hour blisters, and 40-hour blisters. Results represent mean ± SD from n = 7 to 9 individuals.
Expression of neutrophil adhesion molecules over time in the cantharidin skin test. CD11a epitopes (A) and L-selectin (B) expressed on neutrophils in whole blood, 16-hour blisters, and 40-hour blisters. Results represent mean ± SD from n = 7 to 9 individuals.
Western immunoblotting of blister and synovial fluid. (A) Western immunoblotting of CD11a and CD18 subunits in 16-hour blister fluid and a synovial effusion from a patient with inflammatory arthritis, using mAbs 38 and 2E7, respectively. Lysates from purified neutrophils (PMN) are shown as a reference standard for full-length CD11a and CD18 chains. (B-C) Comparison of CD18 blotting using mAb 2E7 and anti–C-terminal antibody. Molecular weight size standards are shown on the left. Arrows on the right indicate the position of full-length (180 kDa and 90 kDa) and truncated (110 kDa and 86 kDa) CD11a and CD18 chains, respectively.
Western immunoblotting of blister and synovial fluid. (A) Western immunoblotting of CD11a and CD18 subunits in 16-hour blister fluid and a synovial effusion from a patient with inflammatory arthritis, using mAbs 38 and 2E7, respectively. Lysates from purified neutrophils (PMN) are shown as a reference standard for full-length CD11a and CD18 chains. (B-C) Comparison of CD18 blotting using mAb 2E7 and anti–C-terminal antibody. Molecular weight size standards are shown on the left. Arrows on the right indicate the position of full-length (180 kDa and 90 kDa) and truncated (110 kDa and 86 kDa) CD11a and CD18 chains, respectively.
Antigenic and ICAM-1 binding properties of shed LFA-1 in blister fluid. (A) An LFA-1–specific ELISA assay was developed that used a variety of CD11a and CD18 antibodies in the capture step, in each case detected with biotinylated anti-CD11a antibody 38. Results represent the mean ± SD concentration of soluble LFA-1 (ng/mL), calibrated using recombinant LFA-1 as a standard. (B) The LFA-1 ELISA was modified with ICAM-1–Fc (5 domain) in the capture step to examine binding by soluble LFA-1 in blister fluid to solid-phase ICAM-1, in the presence and absence of blocking anti-CD18 antibody 6.5E. Recombinant LFA-1–Fc at 5 ng/mL was used as a positive control. Results represent mean ± SD absorbance readings at 405 nm, detected using either biotinylated mAb 38 or IgG2a control antibody.
Antigenic and ICAM-1 binding properties of shed LFA-1 in blister fluid. (A) An LFA-1–specific ELISA assay was developed that used a variety of CD11a and CD18 antibodies in the capture step, in each case detected with biotinylated anti-CD11a antibody 38. Results represent the mean ± SD concentration of soluble LFA-1 (ng/mL), calibrated using recombinant LFA-1 as a standard. (B) The LFA-1 ELISA was modified with ICAM-1–Fc (5 domain) in the capture step to examine binding by soluble LFA-1 in blister fluid to solid-phase ICAM-1, in the presence and absence of blocking anti-CD18 antibody 6.5E. Recombinant LFA-1–Fc at 5 ng/mL was used as a positive control. Results represent mean ± SD absorbance readings at 405 nm, detected using either biotinylated mAb 38 or IgG2a control antibody.
A variety of CD18 antibodies were next used in the capture step to determine whether LFA-1 was shed as an α/β heterodimer. CD18 antibodies mapping to the I-like domain,43 TS1/18 and 6.5E, captured sLFA-1, detectable with anti-CD11a antibody 38 (Figure 6A). This demonstrates that sLFA-1 is shed as a heterodimer with intact I and I-like epitopes. CD18 mAbs MEM-48 and KIM-185, mapping to the epidermal growth factor (EGF)–like modules 3 and 4, respectively,44 did not capture sLFA-1. Nevertheless, sLFA-1 was in an active conformation, since the activation reporter antibodies KIM-127 and mAb 24 both captured the soluble receptor (Figure 6A). A modified assay using ICAM-1–Fc in the capture step showed that sLFA-1 retained ICAM-1 binding activity, and this was specifically inhibited by blocking anti-CD18 antibody 6.5E (Figure 6B). Nonspecific binding to ICAM-1–Fc by the detection antibody was ruled out in control experiments using biotinylated IgG2a in the detection step.
Discussion
Cantharidin-induced skin blisters provide a valuable model in which we have been able to examine how LFA-1 and other adhesion molecules are affected by leukocyte transmigration in a controlled human inflammatory setting. We have shown for the first time that LFA-1 is shed by neutrophils and monocytes following transmigration from blood. Mac-1, detected by mAb ICRF44 mapping to the I domain of CD11b,45 was significantly up-regulated on blister-infiltrated neutrophils and monocytes, consistent with activation of cells during inflammatory recruitment. However, VLA-4 expression was not detected on extravasated neutrophils. This contrasts with results in rodents, which express low levels of neutrophil VLA-4 constitutively,9,46 and the experience of in vitro and in vivo human neutrophil transmigration, which showed induction of VLA-4 on neutrophils following transmigration through cultured human umbilical vein endothelial cells47 or into serum-stimulated suction blisters.32 It has also been proposed that that neutrophil VLA-4 expression in humans may be a feature of sepsis.48
A number of trivial explanations for the loss of surface LFA-1 and concomitant appearance in the blister fluid have been ruled out. Possible membrane ectocytosis was ruled out in 2 ways: first, centrifugation of samples, which has been shown to remove membrane microvesicles,42 did not deplete sLFA-1, and, second, an epitope to the CD11a central region, G25.2, was retained at the cell surface. Apoptosis of neutrophils was addressed through reduced CD16 expression on apoptotic cells,38 and this indicated that neutrophils were 89.9% ± 3.96% viable in blister fluid. The validity of using CD16 staining to identify apoptotic cells in blisters was confirmed with annexin V/PI staining, which indicated more than 90% viability in the granulocyte gate and more than 98% viability in the mononuclear-cell gate (data not shown).
Until now, shedding has been described only for single-chain adhesion molecules. The shedding of an α/β heterodimeric integrin raises the questions of whether both subunits are cleaved and what possible proteases are involved. The approximate cleavage site on the CD11a chain may be estimated from retention of the G25.2 epitope at the cell surface following shedding. G25.2 maps to residues 443 to 654, encompassing the last part of the beta-propeller domain and about half of the thigh domain.40 Thus, the approximate breakpoint must be N-terminal to residue 654. This is consistent with an apparent molecular weight of 110 kDa detected by Western blotting, since the theoretical maximum size of a 654–amino acid shed fragment is 123 kDa, taking into account up to 31 kDa of glycation in the mature CD11a subunit.49 A putative breakpoint N-terminal to residue 654 also offers an explanation for detection of the KIM-127 epitope on shed LFA-1, since the calf region of CD11a, which acts as a constraint in the inactive conformation preventing exposure of KIM-127 epitope,15,16 would be retained at the cell surface following shedding. The shed CD18 subunit was only minimally modified, with an apparent change in electrophoretic mobility from 90 to 86 kDa. It remained conformationally intact up to the tailpiece EGF-like module 2, as shown by capture with KIM-127.44 However, conformation was lost in EGF modules 3 and 4, as shown by lack of capture with MEM-48 and KIM-185 antibodies, respectively, possibly due to altered folding of this membrane proximal region upon shedding. A minimal truncation or loss of conformation of the CD18 C-terminus was also suggested by lack of blotting reactivity to polyclonal anti–β2 subunit C-terminus antibody. Determination of the exact break-point on each of the subunits will require further experiments with the development of an in vitro model for LFA-1 shedding.
Several lines of evidence suggest that the protease(s) involved in LFA-1 shedding is likely to be pericellular. First, LFA-1 was selectively lost from neutrophils and monocytes but not lymphocytes in the same blister fluid, arguing for the existence of a surface protease acting in -cis during the extravasation of neutrophils into skin blisters. Second, cell-free blister fluid when added to freshly isolated neutrophils in vitro did not cause detectable shedding of LFA-1 (data not shown), again arguing for a pericellular sheddase. A number of observations demonstrate that LFA-1 was shed as an active αβ heterodimer. Capture of sLFA-1 by anti-CD18 antibodies TS1/18 and 6.5E, with detection by anti-CD11a antibody mAb 38, proves that sLFA-1, though having a substantially truncated CD11a chain, retains an intact αβ heterodimeric headpiece bearing I and I-like epitopes. An active conformation was suggested by capture with mAb 24, which recognizes a conformational epitope with determinants in the α and β subunits of the headpiece of active LFA-1.17,18 Capture with the anti-CD18 KIM-127 activation reporter antibody and binding to solid-phase ICAM-1 provided further confirmation that LFA-1 was shed as a ligand-binding αβ heterodimer. Whether the release process also causes the shed integrin to alter from a bent to an extended conformation cannot be determined from this evidence.
The possible physiologic significance of LFA-1 shedding remains unknown. However, it is tempting to speculate that shedding may help detach LFA-1 from ICAM-1 on the basolateral side of endothelium following neutrophil transmigration, consistent with the proposed model for transmigration.22 LFA-1 shedding was not observed on lymphocytes, at least not in the cantharidin model, and it therefore remains unknown whether it is relevant to the control of T-cell responses. We have detected shedding as early as 5 hours in the cantharidin skin test (data not shown), at a time when transmigrating neutrophils are adjusting to the subendothelial environment through expression of receptors, such as VLA-2 and VLA-6,32,50,51 for basement membrane proteins. As with shedding of L-selectin,3,4 the net effect of LFA-1 shedding may be to promote subendothelial migration on basement membrane proteins by limiting attachment to ICAM-1. Another possibility is that transient loss of LFA-1 immediately following transmigration, at a time when there is clear evidence for activation of monocytes and neutrophils (ie, increased Mac-1 expression; Figures 2B and 3C), may represent a mechanism to limit inflammatory tissue injury in the subendothelial space. Work with a variety of human in vitro models has shown that cross-linking of neutrophil or monocyte LFA-1 is closely linked to reactive oxygen species release,28,29 which in turn can modify leukocyte adhesion through other receptor pathways.31 Proinflammatory mRNA transcripts are also stabilized in monocytes following LFA-1 engagement.30 A cytoprotective benefit would therefore be predicted to accompany loss of cell-surface LFA-1 due to shedding, which might be further enhanced by the soluble receptor acting as a decoy.
Despite intensive investigation of LFA-1 for more than 20 years, shedding has not previously been described. This may be due to the types of inflammatory model studied and species-specific differences. Cantharidin-induced skin blisters provide a unique and predictable model of the early stages of human cutaneous inflammation in which LFA-1 shedding was detected in 67 of 68 consecutive individuals tested thus far (data not shown). In contrast, LFA-1 shedding was not readily detected in comparable clinical samples. Of 15 synovial aspirates screened in arthritis patients, only one sample revealed soluble LFA-1 by ELISA and Western blotting (a patient presenting with an acute first-time monoarthritis), and even in this patient no loss of membrane-bound LFA-1 could be demonstrated flow cytometrically on synovial infiltrated leukocytes. The difficulty in detecting shed LFA-1 in chronic inflammatory samples may be due to instability of the shed receptor in a protease-rich environment or rapid uptake by ICAM-1 induced on surrounding vasculature and tissue cells as occurs later in the inflammatory process. The problem may be compounded by re-expression of LFA-1 during the inflammatory process, as was noted for both LFA-1 and L-selectin on neutrophils at a later time point (40 hours) in the cantharidin inflammatory model. It was not possible to extend the cantharidin skin test beyond 40 hours to examine whether LFA-1 or L-selectin would become fully reexpressed on neutrophils after the initial shedding event.
In conclusion, by examining neutrophils and monocytes in cantharidin-induced skin blister fluid, we have shown that LFA-1 is shed following transmigration from blood into inflamed tissue. More work will be needed to address the mechanisms and physiologic role of LFA-1 shedding, and whether this process is of relevance to leukocytes other than neutrophils and monocytes. However, shedding of integrins has broad implications for the control of cellular adhesion, communication, and signaling.
Supported by grants from the British Heart Foundation and the Hammersmith Hospital Trustee's Research Committee.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 17, 2006; DOI 10.1182/blood-2005-09-3695.
We would like to thank Dr Richard Day for assisting in the development of the cantharidin skin blister technique and Dr Pete Anning at Scienceposters.co.uk for assistance with figures and article submission.