Comment on Sood et al, page 3173
When trophoblast stem cells differentiate, they acquire an endothelial cell–like thromboregulatory gene expression program and sense, via the expression of protease-activated receptors, the presence of activated coagulation factors.
Pregnancy complications are one of the leading problems in women's health issues: for instance, 9% to 13% of women in the reproductive age group experience 1 clinically recognized pregnancy loss, 5% experience 2 or more losses, and 1% to 2% suffer 3 or more losses. Although several medical causes have been established, up to 50% of cases still remain unexplained.
Clinical studies have shown that maternal inherited and acquired hypercoagulable disorders that promote thrombosis, termed thrombophilia, may increase susceptibility to fetal loss. Among them, the factor V Leiden and prothrombin 20210G>A polymorphisms are associated with an increased risk of spontaneous abortion, if clinical signs occur from the 10th week of the first intended pregnancy.1 However, available data show variable degrees of the association between maternal thrombophilia and adverse pregnancy outcomes, indicating the existence of as yet uncharacterized cofactors acting as risk modifiers.
Humans and mice share a hemochorial placentation model, with zygote-derived trophoblast cells uptaking nutrients from circulating maternal blood. Mouse knock-out models have increased our understanding of the effect of inherited thrombophilia on pregnancy outcome, showing the link with trophoblastic physiology. Thrombomodulin (TM) and endothelial protein C receptor (EPCR) play a crucial role during development—mice lacking either of these molecules die during midgestation.2,3 Restoration of TM expression in the placenta leads TM-null embryos to develop normally during midgestation, and the crucial site wherein EPCR expression is required during development is the placenta. The growth defects of both TM and EPCR knock-out embryos suggested that the protein C system plays a role in regulating trophoblast cell proliferation during development. The impaired trophoblast cell proliferation of the knock-out embryos was linked to altered activation of protease-activated receptors (PARs) at the cell surface, the engagement of PAR-1 by EPCR-bound activated protein C being no longer efficient.4
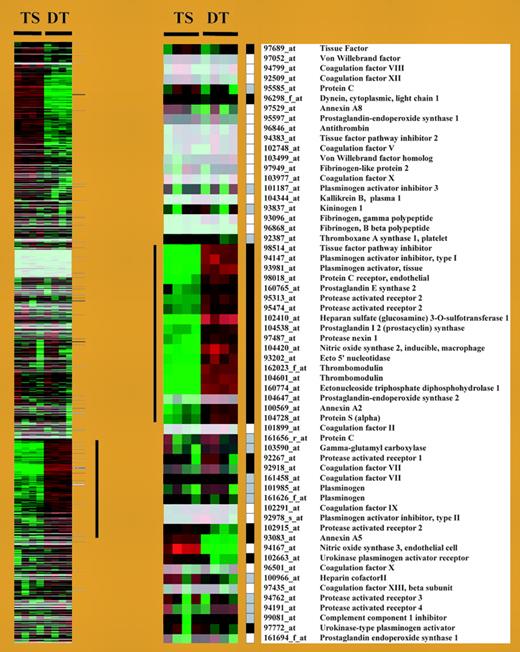
In this issue, Sood and colleagues elegantly show that murine trophoblast cells activate, during differentiation, a gene expression program conferring thromboresistance (see figure). The group of thrombo-regulatory gene products represents a set of candidate genes, transmitted by both parents, which may modulate the risk of adverse pregnancy outcomes experienced by mothers with thrombophilia. The authors also demonstrate that trophoblast cells can sense the presence of activated coagulation factors, which can engage PARs and induce alterations in trophoblast gene expression.FIG1
Cluster display of expression data from TS and DT cells. See the complete figure in the article beginning on page 3173.
Cluster display of expression data from TS and DT cells. See the complete figure in the article beginning on page 3173.
If human and murine physiologies are really similar, the data from Sood et al may open new doors that could prove fascinating and clinically promising. First, the father's genome, as previously suspected,4 may play a part in poor pregnancy outcomes: the NOHA first study,1 which systematically included DNA from the father, is currently working in that direction. Second, it may indicate, among the many women carrying frequent thrombogenic polymorphisms, which really are at risk, thus leading to a more precise definition of women in need of prophylactic treatment,5 to a precise development of these treatments, and maybe to primary prophylaxis. Third, it should clarify the link between thrombophilia and a given type of pregnancy complication. A new area is probably in the offing. ▪