Long-term cures of hemophilia B have been achieved using AAV2 delivering the factor IX gene to the liver of adeno-associated virus (AAV)–naive hemophilic animals. However, the clinical success of this approach requires overcoming pre-existing AAV neutralizing antibodies prevalent in humans. To better define the inhibition of neutralizing antibodies on AAV2-mediated liver transduction, we developed an in vivo passive immunity model. SCID mice were first reconstituted to a defined neutralizing titer with pooled plasma-derived human immunoglobulin. AAV2-FIX vectors then were administered to the liver, and the transduction efficiency was measured by plasma FIX levels. Unexpectedly, AAV2 neutralizing titers lower than 1:10 were sufficient to neutralize 4 to 20 × 1012 vg/kg of AAV2 vectors in vivo, a capacity that was underestimated by in vitro neutralizing assays. We also evaluated strategies to evade neutralization, including the use of alternative delivery routes, infusion parameters, empty capsids, and alternative AAV serotypes 6 and 8. The results indicate that low AAV2 neutralizing titers can be inhibitory to the tested human and primate AAV vectors delivered into the circulatory system. Therefore, novel nonprimate AAV vectors or compartmentalized delivery may offer more consistent therapeutic effects in the presence of pre-existing AAV neutralizing antibodies.
Introduction
Adeno-associated virus (AAV) vectors have reproducibly delivered transgenes in animal models, resulting in successful treatment and cure of numerous clinically relevant diseases. These studies have demonstrated sustained transgene expression without toxicity in mice, rats, dogs, and nonhuman primates.1-11 In addition, several clinical trials using AAV2 vectors have shown excellent safety profiles; however, clear evidence of therapeutic efficacy has not yet been achieved.12,13 A major challenge for AAV-mediated gene therapy is pre-existing AAV-specific antibodies prevalent in the human population resulting from prior natural infections.14-18 The effect of these pre-existing antibodies on AAV gene transfer varies among individuals, depending on actual neutralizing titers, the delivery route, and target organ. Compartments that have limited access to systemically circulating antibodies, such as brain and skeletal muscle, have been transduced successfully in the presence of high circulating neutralizing titers.12,19 Intravascular delivery to target organs such as the liver will likely be more problematic because the circulating antibodies can rapidly bind to antigens at an average Kd of 10-7 to 10-11 M.20 This concept is experimentally supported by the work of other groups who have demonstrated that AAV fails to transduce liver upon re-administration.18,21 The human experience has not yet been fully evaluated since only one clinical trial using liver delivery has been conducted,22 and none have been initiated using intravenous delivery.
The percentage of the human population positive for neutralizing (18%-80%) and nonneutralizing (50%-96%) anti-AAV antibodies varies radically among surveys due to differences in the sensitivity of the serological assays (neutralizing titer assay and enzyme-linked immunosorbent assay [ELISA]), since standardized methods and reagents have not been established.14-18 In addition, this variation also may reflect differences in viral exposure between populations, although the limited data suggest that these population differences may not be as large as once thought.17 However, despite interstudy differences, it is clear that a significant proportion of the population has pre-existing anti-AAV antibodies that likely will inhibit gene transfer as a function of the neutralizing titer. However, the relationship between neutralizing titer and AAV transduction, though critical for successful clinical trial outcomes, has not been thoroughly investigated.
In vitro neutralization assays are not always predictive of in vivo resistance to viral infection from previous vaccination or exposure.23 Any discrepancy probably reflects differences in the mechanism of viral neutralization that occurs in vitro and in vivo. In vitro neutralization is mediated via direct antibody binding of viral capsids and sequestration, induction of viral aggregation, interference with viral attachment to cellular receptors, or prevention of viral uncoating upon cell entry.24 In vivo, in addition to antibody-mediated direct inhibition of viral infectivity, viral particles also are cleared via opsonization. By coating viral particles with antibodies that also bind to Fc receptors on macrophages and neutrophils, both neutralizing and non-neutralizing antibodies stimulate enhanced phagocytosis and clearance of viral particles in vivo.25,26
To date the impact of pre-existing immunity has been modeled by re-administration studies in laboratory animals.27 The limitation of these studies is that the first administration results in very high anti-AAV antibody titers, which presumably would only be seen in humans after a recent exposure. Also, the repertoire and affinity of murine antibodies generated after a single bolus injection of replication-defective AAV vectors in the absence of helper viruses may be different from human antibodies that develop after ongoing exposure to natural wild-type AAV infection. As the circulating titers in healthy adults are often much lower than those in vaccinated animals, it is the impact of such low titers on AAV delivery to the vasculature that needs to be modeled.
To quantify the effect of neutralizing antibodies on AAV-mediated gene transfer in vivo, we have developed a passive immunity model in SCID mice with plasma-derived, pooled human intravenous immunoglobulin (IVIG) as a source of human anti-AAV antibodies. Since human IVIG is isolated from a pool of thousands of individual donor plasma, the repertoire of anti-AAV antibodies present in IVIG should be essentially exhaustive. This strategy allowed us to generate mice with a predefined range of neutralizing titers. Challenging these mice with AAV serotypes has permitted us to quantify the impact on AAV-mediated gene transfer and subsequent transgene expression. In addition, this model facilitates the evaluation of different strategies aimed at escaping neutralization, such as modified delivery parameters, modified vectors, and novel AAV serotypes.
Materials and methods
Vector construction, production, purification, and characterization
The AAV-hFIX vector construct was described previously.28 Briefly, the vector genome contains a liver-specific promoter, a human FIX minigene including the first intron (1.4 kb) and a bovine growth hormone polyadenylation signal flanked by AAV2 inverted terminal repeats (ITRs). The vector genome was packaged into capsids from AAV2, AAV6,29 and AAV830 by triple transfection in 293 cells.31 Vectors were purified on CsCl-density gradients.28 In addition, AAV2 vector also was purified by a chromatography procedure in which empty capsids remain in the final preparation.32 Vector genomes were titered by quantitative real-time polymerase chain reaction (Q-PCR) using primers and probes specific to human FIX sequences and a linearized plasmid DNA standard. AAV particles were quantified by optical density measurement.32
In vitro neutralization antibody assay
Fifty human serum samples were obtained from the Irwin Memorial Blood Center (San Francisco, CA). Test human sera and mouse plasma were heated at 56°C for 30 minutes to inactivate complement, followed by a 2-fold serial dilution in naive mouse serum (Nieffenger, Woodland, CA). 16 μL of diluted test sera was mixed with an equal volume of 1.7 × 108 vg of AAV2-LacZ vector. After 1 hour incubation at 37°C, 7.5 μL of this mixture was used to transduce 2.4 × 104 cells per well of HEK-293 cells seeded 24 hours earlier. After overnight transduction, cells were rinsed with phosphate-buffered saline (PBS) and lysed in 60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 0.27% β-mercaptoethanol, and 0.005% sodium dodecyl sulfate (SDS). The plates were frozen at -80°C and then thawed at 37°C for 15 minutes, respectively. After the addition of the substrate (20 μL of 4 mg of ONPG [o-nitrophenylβ-d-galactopyranoside]) in lysis buffer without SDS and incubation at 37°C for 20 to 60 minutes, the LacZ activity was measured by reading at an absorbance wavelength of 420 nm and analyzed with Softmax Pro software (Molecular Devices, Sunnyvale, CA). Background was defined as the absorbance in untransduced wells. Percent inhibition of AAV-LacZ = 100 – [(Test sample OD-background OD)/(naive mouse serum OD-background OD) × 100]. Percent inhibition of AAV-LacZ versus serum dilution was plotted and fitted using a 4-paramater logistic curve fit in Sigma plot (Systat Software, Point Richmond, CA). The neutralizing titer was determined as the serum dilution at which 50% or higher inhibition occurred.
In vitro neutralizing capacity assay
This assay was used to determine the maximal amount of vector particles neutralized by a given titer of IVIG in vitro. IVIG with starting titer at 1:2930 was diluted in naive mouse serum to generate final neutralizing titers at 1:1, 1:3.3, and 1:10, and AAV2-lacZ vector was diluted in Dulbecco modified Eagle medium to generate the following viral genomes per 0.85 mL (average mouse serum volume): 1.0 × 1010, 4.5 × 1010, and 9.0 × 1010. An equal volume (0.85 mL) of diluted IVIG and vectors were mixed and incubated for 1 hour at 37°C. The transduction and OD420 measurements were performed as described in “In vitro neutralization antibody assay” for the neutralizing titer assay. The neutralizing capacity of a specified titer was defined as the maximal vector dose neutralized by 50% or less.
Passive antibody administration and AAV transduction in SCID mice
SCID (BALB/cByJSmn-Prkdcscid/J;BAIB SCID) or NOD/SCID (NOD/LtSz-Prkdcscid/J; NOD SCID) mice (Jackson Laboratories, Bar Harbor, ME) were reconstituted with human IVIG Panglobulin (100 mg/mL IgG) (ZLB Bioplasma, Berne, Switzerland) diluted in saline by either intravenous or intraperitoneal injection. To evaluate the recovery of IVIG in mice, plasma was collected by retro-orbital bleeding (100 μL into sodium citrate) at 3, 24, and 72 hours; then 1, 2, and 3 weeks after IVIG injection for neutralizing titer assay in pilot experiments.
Immediately prior to AAV-hFIX vector administration, mouse plasma was collected for later determination of the in vivo neutralizing titer. AAV2-, AAV6-, and AAV8-hFIX vectors were delivered intravenously at 5 × 1011 vg/mouse or via intraportal vein or direct liver injection at 1 × 1011 vg/mouse as previously described.33 Mouse plasmas were collected at 1, 2, 4, and 8 weeks after vector injection to measure circulating human FIX levels using a human FIX-specific ELISA (Affinity Biologicals, Ancaster, ON). FIX expression in the IVIG-treated mice was compared with that in control animals, which either had no injection of any antibodies or who received injections of a non–AAV2-neutralizing total rabbit immunoglobulin pool (Pierce Chemical, Rockford, IL). Mice were euthanized 8 to 12 weeks after vector injection, and liver tissues were harvested to determine gene transfer efficiency by Q-PCR specific for human FIX transgene DNA.27
All animal procedures were performed following institutional review and in accordance with “The Guide for Care and Use of Laboratory Animals” (National Institutes of Health, publication 85-23).
Statistical analyses
One- or 2-tailed t tests and 1-way ANOVA with Bonferroni multiple comparison post test were performed using Prism 4 (GraphPad Software, San Diego, CA).
Results
Pre-existing antibodies in the human population
Sera from 50 anonymous healthy human individuals were tested in an in vitro neutralizing assay to determine the AAV2 neutralizing titers. Sixty-two percent tested positive as defined by titers of at least 1:1 (Table 1). However, about half of these positive samples (30%) had neutralizing titers lower than 1:25, which would not be considered significant by many groups who traditionally test sera at starting dilutions of 1:20 or higher.16,18
Characterization of AAV2 antibodies in human IVIG in vitro and in SCID mice
The commercial sources of human IVIG screened for neutralizing antibodies to AAV2 all contain high AAV antibody titers ranging from 1:1043 to 1:3330 (data not shown). However, it should be noted that the IgG concentration in the different IVIGs was between 50 and 100 mg/mL, which is about 10-fold more concentrated than that found in normal serum (5-10 mg/mL). In the following experiments, we used Panglobulin (ZLB Bioplasma) that had an AAV2 neutralizing titer of 1:2930 at an IgG concentration of 100 mg/mL.
First, we determined the kinetics of IVIG passively administered into SCID mice. As the recovery of IVIG in serum postequilibrium with interstitial space was 50% of that injected,34 89 μLof Panglobulin should reconstitute 5 mg of human IgG per mL of mouse plasma, based on 70 mL plasma/kg of body weight,35 producing AAV neutralizing titers of 1:100 to 1:300 in SCID mice. However, the actual titer achieved was 1:30 to 1:100 at 24 hours after injection. Dosing with a 10-fold lower amount of IVIG resulted in approximately a 10-fold lower AAV neutralizing titer; for instance, 8.9 μL of IVIG generated titers at 1:3.3 to 1:10 at 24 hours after injection. In addition, similar titers were achieved by either intravenous or intraperitoneal delivery of IVIG. However, intravenous delivery gave greater consistency among mice. In general, neutralizing titers peaked between 3 hours and 24 hours after intravenous injection and subsequently began to drop. The neutralizing titers were 50% of their peak values between 1 and 3 weeks after administration (data not shown).
Low titers of AAV2 neutralizing antibodies abrogate AAV2-hFIX transduction. SCID mice received intravenous injections of 0.89 and 8.9 mg human IVIG (ZLB, Bioplasma), which resulted in AAV2 neutralizing antibody titer ranges from 4.3 to 12.5 and from 33.3 to 133.3, respectively, as indicated for individual mice. Twenty-four hours later, 5 × 1011 vg of AAV2-hFIX was administered intravenously. Plasma human FIX levels were measured in individual mice 4 weeks after AAV treatment. Error bars indicate SD of replicate samples in ELISA assay.
Low titers of AAV2 neutralizing antibodies abrogate AAV2-hFIX transduction. SCID mice received intravenous injections of 0.89 and 8.9 mg human IVIG (ZLB, Bioplasma), which resulted in AAV2 neutralizing antibody titer ranges from 4.3 to 12.5 and from 33.3 to 133.3, respectively, as indicated for individual mice. Twenty-four hours later, 5 × 1011 vg of AAV2-hFIX was administered intravenously. Plasma human FIX levels were measured in individual mice 4 weeks after AAV treatment. Error bars indicate SD of replicate samples in ELISA assay.
Pre-existing neutralizing antibodies inhibit liver transduction by AAV2-hFIX
We evaluated the effect of pre-existing antibodies on AAV transduction delivered by intravenous injection. Twenty-four hours after IVIG injection, plasma was harvested to determine in vivo neutralizing titers; meanwhile, 5 × 1011 vg of AAV2-hFIX vector was administered. Circulating human FIX levels were measured 2 and 4 weeks later and compared with that in non–IVIG-treated animals.
Four weeks after vector injection, 6 of 7 mice without pre-existing immunity achieved plasma FIX levels of 10 551-20 868 ng/mL, equivalent to 200% to 400% of normal human FIX levels (Figure 1). One mouse had a lower FIX level at 1275 ng/mL that was likely due to misinjection. In contrast, all 7 mice with high neutralizing titers (1:33.3-1:100), and 6 of 7 mice with low titers (1:4-1:12) had no detectable FIX levels; one mouse in the low-titer group expressed low levels of FIX at 121 ng/mL.
The FIX levels correlated with gene transfer as determined by Q-PCR of transgene DNA in mouse liver. Whereas a mean of 1.04 copies per diploid genome was detected in mice not treated with IVIG, mice with low AAV neutralizing titers (1:4-1:12) had a mean of 0.008 copy per diploid genome (130-fold reduction), and those with titers higher than 1:33.3 had no detectable transgene DNA in their liver tissues (Table 2). The sensitivity of the Q-PCR assay was 0.0003 copy per diploid genome. The potent inhibition of low titers of AAV2 neutralizing antibodies in vivo was not predicted by the in vitro neutralizing assay as illustrated in Table 3. For example, at neutralizing antibody titers of 1:3.3 and 1:10, the in vitro assay predicted that only 4.5% and 9% of 5 × 1011 vg vector would be neutralized, respectively. However, experimentally, 100% of input vector was neutralized in vivo. To control for nonspecific neutralization of the AAV-hFIX vectors, we also evaluated FIX expression in SCID mice that received injections of the same concentration of a non-AAV neutralizing total rabbit immunoglobulin pool. This control group had comparable levels of FIX expression to that in the non–IVIG-treated mice (data not shown).
Portal vein and direct liver injection of AAV-hFIX permit some escape from AAV2 neutralization compared with intravenous delivery. SCID mice were reconstituted with IVIG and achieved average AAV2 neutralizing titers as indicated for each group (5-7 mice/group). Mice then received either 5 × 1011 vg/mouse of AAV2-hFIX intravenously (iv) or 1 × 1011 vg/mouse by portal vein (pv) or direct liver (dl) injection. Circulating human FIX levels were measured in mouse plasmas collected at 4 weeks after vector injection and presented as percent of the mean FIX level in the respective non–IVIG-treated group. Error bars indicate SD in each group.
Portal vein and direct liver injection of AAV-hFIX permit some escape from AAV2 neutralization compared with intravenous delivery. SCID mice were reconstituted with IVIG and achieved average AAV2 neutralizing titers as indicated for each group (5-7 mice/group). Mice then received either 5 × 1011 vg/mouse of AAV2-hFIX intravenously (iv) or 1 × 1011 vg/mouse by portal vein (pv) or direct liver (dl) injection. Circulating human FIX levels were measured in mouse plasmas collected at 4 weeks after vector injection and presented as percent of the mean FIX level in the respective non–IVIG-treated group. Error bars indicate SD in each group.
Evaluation of alternative delivery routes and parameters on viral neutralization
Tail vein injection of vector models the level of transduction that could be achieved in the face of maximal vector-antibody mixing. Since the target tissue was the liver, we also evaluated whether injection into the portal vein or directly into the liver parenchyma would better shield vectors from neutralizing antibodies. For these injections, we chose a lower dose of 1 × 1011 vg/mouse because comparable levels of FIX were achieved using either 1 × 1011 vg/mouse by portal vein or 5 × 1011 vg by intravenous injection (2245 ± 758 ng/mL and 5499 ± 2190 ng/mL, respectively, at 2 weeks after injection). We have shown that there is no detectable FIX expression after intravenous delivery of AAV-hFIX, indicating significant vector neutralization by pre-existing anti-AAV2 antibodies at an average titer of 1:7.8 (Figure 2). In the case of portal vein delivery, an average neutralizing titer of 1:3.8 reduced FIX expression to 3.96% ± 1.7% of that in non–IVIG-treated controls; while with direct liver injection, FIX expression, in the presence of an average neutralizing titer of 1:8.2, was reduced to 7.0% ± 7.4% of the controls (Figure 2). Whereas both portal vein and direct liver injection permit some escape of vectors from neutralization, the levels of protection do not differ significantly in comparison with intravenous injection (P > .05). In addition, the direct liver injection resulted in only 20% of FIX expression achieved by the portal vein injection in mice without IVIG (data not shown), indicating less efficient uptake of vector via direct liver injection. However, given the protection seen by the portal vein injection, we further evaluated the effect of altering delivery parameters on vector neutralization. We found that increasing the viral infusion rate from 5 to 22.5 mL/min/kg and/or the infusion volume from 150 to 450 μL provided no additional protection from neutralization (Figure 3).
Pseudotypes AAV6 and AAV8 escape neutralization more effectively than AAV2
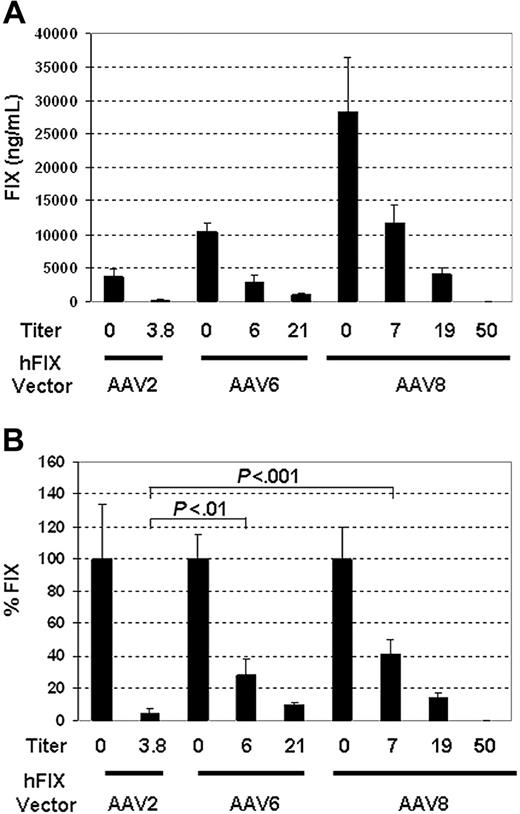
To evaluate whether serotypes other than AAV2 were less neutralized by IVIG, DNA containing the AAV2 ITR and the hFIX expression cassette was packaged into AAV6 or AAV8 capsids. The pseudotyped vectors were delivered by portal vein injection to IVIG-treated mice whose AAV2 neutralizing titers had been determined. As shown in Figure 4A, in the absence of IVIG, AAV2-, AAV6-, and AAV8-hFIX vectors achieved increasingly higher levels of FIX at 3614 ± 1259 ng/mL, 10 359 ± 1187 ng/mL, and 28 377 ± 1076 ng/mL, respectively, at 4 weeks after vector delivery.
Increasing the rate or volume in portal vein injection has no effect on escape of AAV2-hFIX from neutralization. SCID mice (n = 5 per group) were reconstituted with IVIG, resulting in AAV2 neutralizing titers between 1:3.3 and 1:10, followed by portal vein injection of 1 × 1011 vg/mouse of AAV2-hFIX. The rate and volume of delivery are as indicated for each group. Results presented here are mean FIX levels at 4 weeks after vector delivery in each group. Error bars indicate SD.
Increasing the rate or volume in portal vein injection has no effect on escape of AAV2-hFIX from neutralization. SCID mice (n = 5 per group) were reconstituted with IVIG, resulting in AAV2 neutralizing titers between 1:3.3 and 1:10, followed by portal vein injection of 1 × 1011 vg/mouse of AAV2-hFIX. The rate and volume of delivery are as indicated for each group. Results presented here are mean FIX levels at 4 weeks after vector delivery in each group. Error bars indicate SD.
In contrast to AAV2-hFIX–treated mice, which lost 96% ± 3% of their expression at neutralizing titers as low as 1:3.8, AAV6 and AAV8 pseudotyped vectors retained 28% ± 10% and 41% ± 9% of FIX expression at an average neutralizing titer of 1:6 and 1:7, respectively (Figure 4B). Both serotypes are significantly more resistant to neutralization than AAV2 (P < .001 by 1-way ANOVA with Bonferroni post test). Even at a mean titer of 1:20, AAV6 and AAV8 still retained 9% ± 2% and 14% ± 3% of FIX expression, respectively (Figure 4B). However, as pre-existing neutralizing titers were increased to between 1:33.3 and 1:100, FIX expression from either vector became undetectable, indicating complete neutralization of these vectors (Figure 4A).
AAV6 and AAV8 pseudotypes escape neutralization more efficiently than AAV2 via portal vein delivery. Mean AAV2 neutralizing titers generated by IVIG in SCID mice (n = 5 per group) are as indicated for each group. At 24 hours after IVIG administration, 1 × 1011 vg/mouse of AAV-hFIX with specified pseudotypes were delivered via the portal vein. hFIX expression was measured at 4 weeks. (A) Mean FIX levels (ng/mL) from each group. (B) Percent of mean FIX levels in IVIG-treated group compared with that in non–IVIG-treated controls. P values from Bonferroni post test in one-way ANOVA are listed above the columns. Error bars indicate SD in each group.
AAV6 and AAV8 pseudotypes escape neutralization more efficiently than AAV2 via portal vein delivery. Mean AAV2 neutralizing titers generated by IVIG in SCID mice (n = 5 per group) are as indicated for each group. At 24 hours after IVIG administration, 1 × 1011 vg/mouse of AAV-hFIX with specified pseudotypes were delivered via the portal vein. hFIX expression was measured at 4 weeks. (A) Mean FIX levels (ng/mL) from each group. (B) Percent of mean FIX levels in IVIG-treated group compared with that in non–IVIG-treated controls. P values from Bonferroni post test in one-way ANOVA are listed above the columns. Error bars indicate SD in each group.
It is important to note that even in the face of a mean anti-AAV2 titer of 1:19, AAV8-hFIX vector achieved FIX levels (4059 ± 969 ng/mL) comparable to that by AAV2 (3614 ± 1259 ng/mL) in the absence of any neutralizing antibody (Figure 4A). AAV6-hFIX expressed approximately 4-fold lower levels of FIX at 959 ± 181 ng/mL compared with AAV8 at the same titer (Figure 4A).
In addition, we evaluated resistance of AAV2-, AAV6-, and AAV8-hFIX vectors to neutralization following intravenous delivery (Figure 5). Whereas 5 × 1011 vg of AAV2 vector yielded no detectable FIX expression at an average neutralizing titer of 1:8, AAV6 and AAV8 retained 92% ± 26% and 64% ± 17% of FIX expression, respectively, at comparable titers (Figure 5B). Thus, both AAV6 and AAV8 vectors demonstrated significantly higher levels of protection from neutralization than AAV2 (P < .001), although they did not differ significantly from each other (P > .05). Impressively, even at an average neutralizing titer as high as 1:131, AAV8 still maintained significant levels of FIX expression at 17 391 ± 13 117 ng/mL, which is 26% ± 19% of non-IVIG control (Figure 5A,B). AAV8 vectors were completely neutralized when the neutralizing titer was raised to 1:255 (Figure 5A,B). Overall, proportionally higher neutralizing titers are required to neutralize AAV8 vectors injected intravenously than via the portal vein, because 5-fold higher vector dose was administered intravenously. In summary, AAV6 and AAV8 achieve both higher levels of FIX expression and increased resistance to pre-existing AAV2 neutralizing antibodies under conditions where AAV2 would be completely neutralized.
AAV6 and AAV8 pseudotypes escape neutralization more efficiently than AAV2 via intravenous delivery. Mean AAV2 neutralizing titers generated by IVIG in SCID mice (n = 5 per group) are as indicated for each group. Twenty-four hours after IVIG administration, 5 × 1011 vg/mouse of AAV-hFIX with specified pseudotypes were delivered intravenously. hFIX expression was measured at 4 weeks. (A) Mean FIX levels (ng/mL) from each group. (B) Percent of mean FIX levels in IVIG-treated group compared witih that in non–IVIG-treated controls. P values from Bonferroni post test in one-way ANOVA are listed above the columns. Error bars indicate SD in each group.
AAV6 and AAV8 pseudotypes escape neutralization more efficiently than AAV2 via intravenous delivery. Mean AAV2 neutralizing titers generated by IVIG in SCID mice (n = 5 per group) are as indicated for each group. Twenty-four hours after IVIG administration, 5 × 1011 vg/mouse of AAV-hFIX with specified pseudotypes were delivered intravenously. hFIX expression was measured at 4 weeks. (A) Mean FIX levels (ng/mL) from each group. (B) Percent of mean FIX levels in IVIG-treated group compared witih that in non–IVIG-treated controls. P values from Bonferroni post test in one-way ANOVA are listed above the columns. Error bars indicate SD in each group.
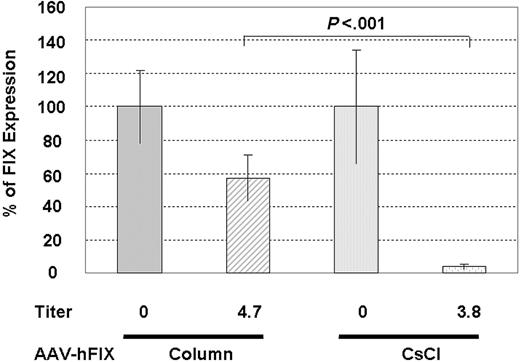
Column-purified AAV2-hFIX escapes neutralization more efficiently than CsCl-purified vector. SCID mice without or with IVIG treatment received injections via the portal vein of 1 × 1011 vg/mouse column- or CsCl-purified AAV2-hFIX. The column-purified vector has a ratio of total particles to genome-containing particles of 12:1, whereas the CsCl vector preparation contains only full vectors. FIX levels were measured 4 weeks after vector injection. P value from unpaired 2-tailed t test is shown above the bar.
Column-purified AAV2-hFIX escapes neutralization more efficiently than CsCl-purified vector. SCID mice without or with IVIG treatment received injections via the portal vein of 1 × 1011 vg/mouse column- or CsCl-purified AAV2-hFIX. The column-purified vector has a ratio of total particles to genome-containing particles of 12:1, whereas the CsCl vector preparation contains only full vectors. FIX levels were measured 4 weeks after vector injection. P value from unpaired 2-tailed t test is shown above the bar.
Less neutralization of AAV2-hFIX vectors in the presence of empty AAV2 particles
AAV2 vectors purified by chromatography contain empty capsids at approximate ratios of 10:1 to 30:1 (total particles to genome containing particles).32 In contrast, AAV2 vectors purified by CsCl density gradient contain undetectable levels of empty particles. Therefore, at the same dose by vector genome, column-purified vectors contain substantially more AAV2 capsids compared with CsCl-purified vectors, which should absorb a significant proportion of pre-existing AAV2 antibodies.
To test this hypothesis, we injected 1 × 1011 vg of column-purified AAV2-hFIX vector by portal vein into SCID mice containing average neutralizing titers of 1:4.7 ± 1.0. This vector preparation contained a ratio of total particles to full particles of 12:1, so the actual dose injected was 1.2 × 1012 total particles. At 4 weeks after injection, FIX expression in the IVIG-treated animals was 57% ± 16% of that in the non–IVIG-treated mice, which is significantly higher than the 4.0% ± 1.7% of FIX expression retained by CsCl-purified vector in the presence of neutralizing titers of 1:3.8 (Figure 6) (P < .001).
Involvement of innate immunity in vector clearance in vivo
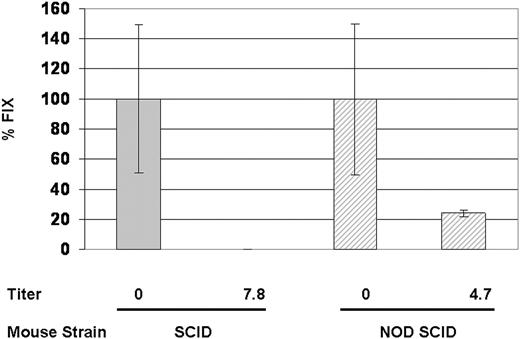
To determine if the innate immune system plays a role in vector clearance, we set up the passive immunity model in NOD/SCID mice. NOD/SCID mice not only lack both B and T cells, but also have impaired functions in natural killer cells, macrophages, and antigen-presenting cells. They also are deficient in the C5 peptide and therefore cannot generate both the classical and alternative pathways of hemolytic complement activation.36 The percentage of AAV neutralization was compared in IVIG-treated SCID or NOD/SCID mice. 4 weeks after intravenous injection of 5 × 1011 vg of AAV2-hFIX, IVIG-treated NOD/SCID mice retained approximately 25% of hFIX expression compared with that in non–IVIG-treated NOD/SCID mice (Figure 7). In contrast, no hFIX expression was detected in SCID mice in the presence of low neutralizing titers (Figure 7), suggesting that although antibody-mediated neutralization is involved in the bulk of vector neutralization, innate immunity also plays a role in vector clearance.
Increased transduction in NOD/SCID mice suggests a role for innate immunity in viral clearance. SCID and NOD/SCID mice (n = 5 per group) with or without IVIG treatment received intravenous injections of 5 × 1011 vg/mouse AAV2-hFIX. FIX levels were measured at 4 weeks after vector injection.
Increased transduction in NOD/SCID mice suggests a role for innate immunity in viral clearance. SCID and NOD/SCID mice (n = 5 per group) with or without IVIG treatment received intravenous injections of 5 × 1011 vg/mouse AAV2-hFIX. FIX levels were measured at 4 weeks after vector injection.
Discussion
Clinical implications of neutralizing AAV at low titers
The results from this study imply that clinically successful liver-directed gene therapies by AAV vectors may be more difficult than previously assumed, due to the presence of pre-existing antibodies to AAV capsids. While it was expected that pre-existing AAV neutralizing antibodies would decrease the efficacy of AAV vectors, the severity of this problem has not been adequately appreciated. The present study demonstrates that neutralizing antibodies at titers lower than 1:10 can abrogate liver transduction by high doses of AAV2-hFIX at 4 × 1012 vg/kg delivered via portal vein or 2 × 1013 vg/kg delivered intravenously in mice. Considering that a significant proportion (68%) of the 50 healthy individuals surveyed by us had AAV neutralizing titers, including the 32% of the population that had titers lower than 1:20, a majority of the population may be recalcitrant to liver-targeted AAV gene therapy.
It is of interest to note that the percentage of the human population positive for AAV neutralizing antibodies was much higher in this study compared with other studies.4-7 It is possible that this variation may reflect differences in viral exposure among populations; however, a worldwide survey indicated less significant interracial or geographic differences as previously thought.17 On the other hand, our findings may be attributed to the improved sensitivity of our assay. Chirmule et al16 determined that 32% of the population was positive for AAV neutralizing antibodies by using a starting dilution of 1:20 for human sera. However, 96% were positive for total anti-AAV antibodies as measured by ELISA. Similarly, Moskalenko et al18 found that only 18% of the population had AAV neutralizing antibodies, using a starting dilution of serum at 1:100. In contrast, we performed our neutralizing titer assay starting with undiluted sera and were able to detect AAV neutralizing titers as low as 1:1 in 62% of the population. However, only 32% of the population had titers at 1:25 or higher. A potential caveat of using undiluted sera in our in vitro assay is that the concentrated serum proteins, though nonspecific, may interfere with transduction of the target cells by AAV-LacZ vector, thus resulting in overestimation for samples, which have marginal titers around 1:1. Therefore, to minimize the impact of any nonspecific inhibition of undiluted sera, the neat mouse serum was used as the positive control to determine the relative levels of inhibition by the test sera. Clearly, if in vivo neutralization can occur at titers lower than 1:10, assays must be sufficiently sensitive to measure titers in this range.
Results of this study represent the average level of AAV neutralization in the human population because IVIG, which is purified from thousands of individuals, represents the repertoire of anti-AAV antibodies with heterogeneous specificities and affinities in the population. Therefore, one would expect variations among individuals despite their identical titers, due to their differences in antibody affinities and epitope specificities that may be either underrepresented or overrepresented in the pooled IVIG preparation. These factors may alter the degree of individual responses to AAV2 vectors. Indeed, when we used sera from a single donor in SCID mice, we observed about 84% AAV2 neutralization at a titer of 1:12, compared with 96% at a lower titer of 1:3.8 by IVIG (data not shown). Nevertheless, the individual serum still significantly inhibited vector transduction. Collectively, the results suggest predictive and accurate dosing will be difficult to achieve in humans unless the neutralization effect can be largely or entirely circumvented.
In vitro assays underestimate the neutralization capacity of AAV-specific antibodies in vivo
The neutralization capacity in vivo was underestimated by the in vitro neutralizing titer or capacity assays. However, a number of different groups have previously documented discrepancies between in vitro and in vivo neutralizing capacities of antibodies.23,25 It may be attributed at least in part to direct viral neutralization as well as antibodies interacting with other components of the immune system in vivo, such as complement, phagocytic cells, and natural killer cells. This combination of activities serves to synergize viral clearance via both humoral and cellular immune mechanisms. Thus, the in vivo effect of antibody depends on not only the antigen-binding variable region (Fab), but also the constant region (Fc) that interacts with complement and cellular Fc receptors. This was the case for a protective monoclonal antibody, which after pepsin digestion lost its ability to protect neonatal mice from foot-and-mouth disease virus (FMDV).25 Consistent with this notion, we also have shown that AAV was cleared less efficiently by passively administered IVIG in the NOD/SCID strain compared with the SCID strain of mice. NOD/SCID mice, in addition to lacking both B and T cells, have impaired functions in natural killer cells, macrophages, and antigen-presenting cells, as well as complement protein C5, all innate immunity functions.
Alternatively, it also is possible that additional viral antigens become unmasked in vivo than in vitro, permitting interactions with antibodies that are non-neutralizing in vitro, as indicated for monoclonal antibodies against murine cytomegalovirus (MCMV) infection in mice.23
Evaluation of strategies to evade neutralization
Of the strategies we have evaluated, direct liver and portal vein injection did not significantly improve AAV escape from neutralization compared with intravenous delivery. Neither did further increasing the delivery rate to 22 mL/kg/min or volume to 26% of the blood volume improve vector escape beyond that with our standard portal vein conditions in mice. Normally, mice receive intraportal vein injection of vectors in a volume that is about 8% of the total blood volume and at 5 mL/min/kg, which is 50-fold higher than the delivery rate that was used in our human clinical trial.22 Therefore, it is unlikely that simply altering the delivery routes and parameters will significantly protect vectors from neutralization in humans.
On the other hand, the presence of empty capsids, which served as decoys, significantly reduced neutralization of vectors. This result implies that antibody absorption prior to vector administration may be an effective approach to reduce vector neutralization. The principle of antibody absorption has been demonstrated in adenoviral studies, where chromatography columns coupled with adenoviral capsid proteins (hexon, penton, and fiber) reduced neutralizing antibodies in human sera in vitro37 and ex vivo.38 Antibody absorption using empty AAV capsids coupled with immunopheresis could potentially reduce total circulating anti-AAV antibody; however, a significant proportion (50%) of IgG is located in interstitial spaces, which are not accessible by these procedures.38
Alternative serotypes of AAV less inhibited by IVIG
Alternative AAV serotypes, such as AAV6 and AAV8, are potentially attractive replacements for AAV2. Both AAV6 and AAV8 vectors achieved higher levels of liver transduction compared with AAV239 and have less cross-reactivity to anti-AAV2 antibodies. AAV8 originally was isolated from nonhuman primates; however, sequences closely related to AAV8 also have been isolated from a variety of human tissues.40 The presence of AAV8 neutralizing antibodies in the human population has been reported to be markedly less than those against AAV2 (3.8% vs 20%).30 Although we have not determined the neutralizing titer of IVIG to AAV6 and AAV8, AAV6 and AAV8 share 83% homology in nucleotide sequences of viral capsids with AAV2,40 which likely confers the observed cross-reactivity, assuming AAV2 was the original immunogen.
In this study, we demonstrated that AAV6 and AAV8 are significantly more resistant to neutralization by IVIG than AAV2. Thus, these vectors could potentially treat a significantly larger population than AAV2 (68% vs 40%), which is encouraging though not sufficient if treatment of the entire population is desired. Given the cross-reactivity of neutralizing antibodies in humans and the prevalence of natural infection by various AAV serotypes in humans, it may be necessary to extend the search for novel vectors beyond primates into species that are phylogenetically more distant from humans.41 An alternative strategy employed by a number of groups has been to use site-directed mutagenesis to disrupt neutralizing epitopes on AAV capsids,42,43 which has achieved limited success so far.
In human clinical trials using the same vector, higher titers of anti-AAV2 anecdotally appeared to abrogate transgene expression from liver, but the numbers are too small to make conclusions.22 However, in subjects with low anti-AAV2 titers, where some hepatocyte transduction would be expected, a cell-mediated immune response directed against AAV2 capsid peptides was detected. This T-cell–mediated response putatively destroyed FIX-producing hepatocytes that displayed AAV2 capsid peptides on the cell surface, resulting in the loss of maximally 12% factor IX circulating levels to baseline (< 1%) over several weeks. Thus, even if anti-AAV2 antibodies were eliminated as an issue, the T-cell response, not detected in any animal models, still may require clinical intervention.22
In summary, we have demonstrated that pre-existing immunity is a significant hurdle for liver transduction by AAV vectors delivered into the vasculature. Our passive immunity mouse model has shown that the neutralizing capacity of low titers of anti-AAV2 antibodies in vivo is significantly greater than predicted by the in vitro neutralizing assay. Based on these observations, we believe that the passive immunity model has greater relevance than current in vitro assays for modeling neutralization in humans. We currently are using this model to evaluate novel nonprimate AAV vectors as well as delivery into tissue compartments having less contact with blood that may afford greater escape from neutralization.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-08-3229.
Supported in part by Bayer HealthCare.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Gibrail Haniff and Cheryl Pater for their help with animal surgery.
Dr Scallan's current address is West Coast Biologicals, 600 Townsend St, San Francisco, CA 94103; e-mail: ciaran_scallan@yahoo.com. Dr Couto's current address is Benitec, 2375 Garcia Ave, Mountain View, CA 94043; e-mail: lcouto@benitec.com. Dr Pierce's current address is Bayer HealthCare, 800 Dwight Way, Berkeley, CA 94170; e-mail: Glenn.Pierce.B@bayer.com.