A novel recombinant interleukin-7/hepatocyte growth factor β-chain (IL-7/HGFβ) hybrid cytokine was constructed as a single chain (sc) composed of IL-7 and HGFβ connected by a flexible linker. Unlike recombinant (r) IL-7, which stimulated pro-B cells and pre-B cells only, scIL-7/HGFβ stimulated the proliferation of pre-pro-B cells, common lymphoid progenitors (CLPs), and colony-forming unit (CFU)-S12 in cultures of IL-7-/- mouse BM cells. When injected in vivo, 3- to 4-fold more splenic B-lineage cells appeared in recipients of bone marrow (BM) cells from the scIL-7/HGFβ-stimulated cultures than from rIL-7-stimulated cultures. Moreover, on a per-cell basis, scIL-7/HGFβ culture-generated cells produced 16- to 20-fold more BM and splenic B-lineage cells than did normal BM cells. Antibody blocking, receptor phosphorylation, and confocal microscopy demonstrated that scIL-7/HGFβ signals though both the IL-7 and HGF (c-Met) receptors, which form IL-7R/c-Met complexes on the surface of CLPs and pre-pro-B cells. In addition, the IL-7Rα chain, γc chain, and c-Met were coisolated from purified CLPs and pre-pro-B cells on scIL-7/HGFβ affinity gels, indicating that they are major components of the IL-7/HGFβ receptor. Hence, the present results demonstrate that the IL-7/HGFβ hybrid cytokine efficiently and selectively stimulates the most primitive B-lineage precursors in BM by inducing juxtacrine interactions between the IL-7 and c-Met receptors.
Introduction
Cytokines and growth factors play critical roles in regulating the proliferation, differentiation, and survival of hematopoietic and lymphoid cells. Interleukin-7 (IL-7), originally described as a pre-B cell growth factor, is a nonredundant cytokine that has diverse effects on the hematopoietic and immunologic systems.1-3 Although it has been proposed that IL-7 is capable of acting on primitive B220- B cell progenitors alone or in the presence of Flt-3L and/or c-kitL,4-7 most investigators have concluded that the principal B-lineage targets for IL-7 are pro-B cells and pre-B cells.6,8-14 This view is supported by the observations that neither the administration of IL-7 nor the deregulated overexpression of IL-7 appears to affect pre-pro-B cell development or expansion in vivo,15-17 and that STAT 5 activation enhances pro-B cell and pre-B cell, but not pre-pro-B cell expansion.18
In previous experiments, we identified a naturally occurring heterodimeric form of IL-7 consisting of a self-assembling complex of IL-7 and the beta chain of hepatocyte growth factor (HGF),19-21 a pleiotropic cytokine that regulates parenchymal cell growth, motility, and morphogenesis.22-24 HGF has been reported to regulate hematopoiesis in mouse fetal liver and adult bone marrow, where it apparently can substitute for the stem cell factor (SCF) and c-kit system.25,26 However, its role in the regulation of lymphopoiesis is unclear.27-29 Therefore, it is of interest that the IL-7/HGFβ hybrid cytokine was found to selectively induce proliferation and differentiation of pre-pro-B cells in rat BM, and, by up-regulating IL-7Rα chain and cμ expression, to enable pro-B cells to respond to monomeric IL-7.30 These results have been duplicated using complexes of recombinant (r) IL-7 and rHGFβ formed in the presence of heparan sulfate-derived oligosaccarides.21 However, these complexes proved to be much less stable than was native IL-7/HGFβ, possibly due to incomplete glycosylation. Consequently, it proved difficult to purify sufficient IL-7/HGFβ for in vivo and large-scale in vitro studies.
In order to produce large quantities of stable hybrid cytokine, we have constructed a novel Il-7/HGFβ DNA connected by a flexible linker. After expression in several systems, the resulting recombinant single-chain (sc) IL-7/HGFβ protein was shown in prelimary studies to be active in cultures of rat bone marrow (BM) cells and thymocytes, much like native IL-7/HGFβ. In the present study we formally tested the functional properties of scIL-7/HGFβ in primary cultures of mouse BM cells in order to: (1) identify the cellular targets of this hybrid cytokine; (2) determine the lymphopoietic potential of culture-generated cells in adoptive recipients; and (3) identify the receptor for the IL-7/HGFβ hybrid cytokine. The results demonstrate that scIL-7/HGFβ, unlike IL-7, stimulates the proliferation of day-12 spleen colony-forming units (CFU-S12) and common lymphoid progenitors (CLPs), as well as pre-pro-B cells. Furthermore, cells from scIL-7/HGFβ-stimulated cultures generated B-lineage cells in vivo more effectively than did those from IL-7-stimulated cultures. Lastly, scIL-7/HGFβ was found to coordinately signal through both the IL-7 and HGF (c-Met) receptors, which formed complexes on the cell surface. We propose that it is this juxtacrine property of the IL-7/HGFβ hybrid cytokine that promotes complimentary signaling required for the proliferation of contact-dependent B-cell precursors, which express only low levels of the IL-7Rα chain.
Materials and methods
Animals
129XB6F2 IL-7-/- and IL-7+/+ mice (generously provided by Dr Richard Murray, DNAX Research Institute of Cellular and Molecular Biology, Palo Alto, CA) and Lewis strain rats were used as donors and/or recipients of BM lymphoid precursor cells and thymocytes.
Cytokines and antibodies
Recombinant IL-7 and goat polyclonal antibodies (Abs) against mouse IL-7, IL-7Rα, γc chain, and c-Met (R&D Systems, Minneapolis, MN); goat and rabbit polyclonal antibodies against human and mouse HGFβ, rabbit anti-IL-7, and horseradish peroxidase (HRP)-linked anti-goat and anti-mouse immunoglobulin G (IgG) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); HRP anti-rabbit IgG antibody (Amersham Biosciences, Piscataway, NJ); phycoerythrin (PE), biotin-conjugated, and unconjugated mouse monoclonal antibodies (mAbs) against rat HIS24 (CD45R-B220), HIS17 (CD43), HIS50 (HSA), and fluorescein isothiocyanate (FITC), PE, biotin, and allophycocyanin (APC)-conjugated anti-mouse B220, anti-HSA and anti-CD43 Abs (BD Biosciences, San Diego, CA); FITC and APC anti-IL-7Rα, APC anti-mouse CD4, and PE anti-AA4.1 (Bioscience, San Diego, CA); Alexa fluor 594-labeled anti-goat IgG (Molecular Probes, Eugene, OR); anti-CD19, B220, and PE MicroBeads (Miltenyi Biotec, Auburn, CA); anti-Jak3 and phosphotyrosine (4G10) (Upstate Biotechnology, Lake Placid, NY); and anti-phospho-c-Met (Biosource, Camarillo, CA).
Thymidine incorporation
Thymocytes or culture-generated BM lymphoid cells were seeded in triplicate into 96-well plates, and 0.074 MBq (2 μCi) [3H] thymidine was added to each well 12 hours before completion of the 72-hour incubation period. Incorporation of [3H] thymidine was determined by liquid scintillation spectroscopy.
Brdu incorporation
Brdu solution (10 μL of 1 mM; BD Biosciences) was added per milliliter of cultured BM cells. The treated cells were incubated at 37°C for 45 minutes, and stained with fluorescent antibodies for cell-surface markers. After fixation and treatment with DNase, the cells were stained with APC-labeled anti-Brdu antibody and analyzed by flow cytometry.
Immunomagnetic cell separation
BM cells were stained with CD19 MicroBeads for 15 minutes at 4°C, washed, and then applied to a magnetic-activated cell sorter (MACS) magnetic column. The CD 19- cells were reacted with anti-B220 MicroBeads, and the CD 19-B220+ cells were isolated by immunomagnetic separation (IMS). Alternately, CD 19- cells were stained with PE-anti-AA4.1 Ab and anti-PE MicroBeads, and the CD 19-AA4.1+ cells were collected.
Confocal microscopy
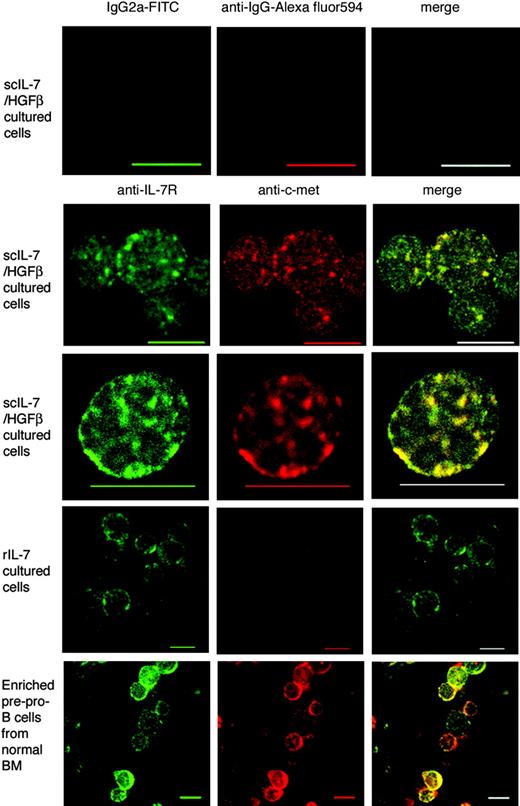
Cytospin preparations of culture-generated BM lymphoid cells or enriched pre-pro-B cells from fresh BM were fixed with 4% paraformaldehyde and stained with FITC-labeled rat anti-IL-7Rα Ab or FITC-labeled rat IgG2a (isotype control) and purified goat anti-c-Met or goat IgG (isotype control) developed with Alexa fluor 594-labeled anti-goat IgG. The cells were then observed under a Zeiss LSM510 Meta laser scanning confocal microscope equipped with a Plan-Apochromat 63 ×/1.4 numeric aperture objective (Carl Zeiss, Thornwood, NY). Images were processed using Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Western blotting
Samples were mixed with 2 × sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE), and then transferred to Immobilon-P membranes (Millipore, Bedford, MA). After blocking with 5% blocking reagent, the membranes were incubated with primary antibodies, HRP-labeled secondary antibodies, and then developed with enhanced chemiluminescence (ECL; Amersham Biosciences).
Construction and expression of a single-chain murine IL-7/HGFβ protein in baculovirus-insect cell expression system
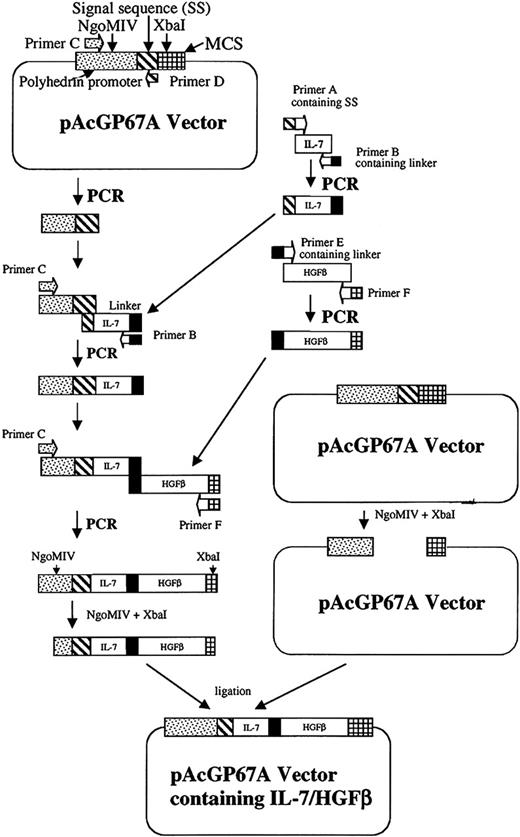
The cDNA encoding murine IL-7 was generated from cultured thymus stromal cells of IL-7+/+ mice, and amplified with primers specifying the mature protein-coding region. Baculovirus transfer vector pAcGP67A (BD Biosciences) containing the gp67 secretion signal sequence was used for the expression and secretion of a scIL-7/HGFβ protein in insect cells (Figure 1). In order to insert IL-7 into the vector and to construct IL-7 and HGFβ, DNA connected by a flexible linker encoding (Gly4Ser) 2,31 IL-7 DNA was amplified with primers (Table 1) containing the 3′ end of gp67 secretion signal sequence (primer A) and the linker sequence (primer B). The gp67 secretion signal sequence and a part of the polyhedrin promoter (containing the NgoMIV site) were amplified from the vector with primers C and D. The polymerase chain reaction (PCR) products of IL-7 and the signal sequence (SS-IL-7) were combined and subjected to an additional round of PCR with primers C and B. Because the 5′ end of the IL-7 PCR product overlaps the 3′ end of the signal sequence, the IL-7 DNA was seamlessly fused to the signal sequence after overlap extension PCR. The cDNA encoding murine HGFβ was amplified from plasmid DNA with primers containing the linker (primer E), and stop codon and XbaI site (primer F). SS-IL-7 and HGFβ DNA were combined and subjected to an overlap extension PCR by primers C and F. As the linker sequences in the 3′ end of SS-IL-7 and the 5′ end of HGFβ overlap, an SS-IL-7 linker IL-7/HGFβ DNA was constructed. The construct was digested with NgoMIV and XbaI, ligated into the NgoMIV/XbaI sites of the transfer vector, and transformed into Escherichia coli DH5α cells. The plamid DNA was purified and sequenced. Sf9 insect cells were cotransfected with the transfer vector and BaculoGold linearized baculovirus DNA (BD Biosciences) to construct a recombinant baculovirus containing the SS-IL-7 linker IL-7/HGFβ DNA via homologous recombination. The recombinant baculovirus was plaque selected, and virus banks were generated according the instruction manual (BD Biosciences). Sf9 insect cells were then infected with the recombinant baculovirus to produce the scIL-7/HGFβ protein. The highest protein expression level was achieved at a multiplicity of infection (MOI) of 2 in suspension culture (26°C for 96 hours) using SF900II serum-free medium (SFM; Invitrogen, Carlsbad, CA) without protease inhibitor.
Cloning strategy for ligation of the IL-7/HGFβ coding sequences into baculovirus transfer vector pAcGP67A. The gp67 secretion sequence, IL-7, linker, and HGFβ DNA are constructed by overlapping PCR as described in “Materials and methods.”
Cloning strategy for ligation of the IL-7/HGFβ coding sequences into baculovirus transfer vector pAcGP67A. The gp67 secretion sequence, IL-7, linker, and HGFβ DNA are constructed by overlapping PCR as described in “Materials and methods.”
Expression of the single-chain IL-7/HGFβ protein in mammalian and yeast expression systems
The IL-7 linker HGFβ (IL-7/HGFβ) DNA was also subcloned into mammalian expression vector pSecTag2A containing signal IgK sequence (Invitrogen) with AscI/XhoI sites (primers G, H). The plasmid DNA was transfected into Chinese hamster ovary (CHO) cells.
To express and secrete the single-chain IL-7/HGFβ protein in a yeast expression system, a yeast expression vector was modified by insertion of a Saccharomyces cerevisiae (SC) α-factor secretion signal (cut with HindIII/XbaI) from pPIC6αA vector into HindIII/XbaI sites of YES2 (Invitrogen). IL-7/HGFβ DNA was subcloned into the modified vector with XhoI/XbaI sites (primers I and F; Table 1). The vector containing the IL-7/HGFβ DNA was transformed into competent INVSc1 cells with SC. Easy comp transformtion kit (Invitrogen) and the transformants were selected with SC-U selection plates. The INVSc1 cells were cultured in SC-U medium containing 2% glucose or raffinose and then induced by 2% galactose for another 4 to 22 hours.
Protein production and purification
The culture supernatant from the recombinant baculovirus infected sf9 cells was concentrated by a Prep/scale-tangential flow filter (TFF) cartridge with 10 kDa molecular weight (MW) cut-off (Millipore, Bedford, MA) and diafiltered into washing buffer (30 mM Na2PO4, pH 7), The samples were then applied to serially linked columns of DEAE and CM sepharose (Amersham Biosciences). After washing, the linked columns were separated, and proteins were eluted from each by stepwise NaCl gradient from 25 mM to 300 mM in the washing buffer. The 200 to 300 mM CM eluates and the 37.5 to 100 mM DEAE eluates were pooled and loaded respectively on a Sephacryl S-200 column (Amersham Biosciences) pre-equilibrated with 30 mM Na2PO4 and 250 mM NaCl (pH 7). Fractions were collected and analyzed for IL-7/HGFβ protein expression by Western blotting and thymocyte stimulating activity. Proteins were quantified by protein assay (Bio-Rad, Hercules, CA), using bovine serum albumin as a standard. Yields of up to 1 mg purified scIL-7/HGFβ per liter of culture supernatant were obtained.
BM lymphoid cell culture and flow immunocytometric analysis
Rat and mouse femoral BM cells were collected by flushing with cold RPMI-1640, and the erythrocytes were lysed with 0.165 M NH4Cl. Nucleated cells (2 × 106) in 2 mL RPMI-1640 containing 5% lot-selected, defined fetal bovine serum (FBS) and 5 × 10-5 M 2-mercaptoethanol (2-ME) were incubated in 35-mm diameter culture plate wells at 37°C in 5% CO2 in the presence of rIL-7 and/or scIL-7/HGFβ. Fourteen to 19 days later, the nonadherent cells were harvested for phenotypic analysis. The cell samples were analyzed by CELL Quest on a FACScan or FACSCalibur flow cytometer (all from Becton Dickinson Immunocytometry Systems, San Jose, CA).
Immunoprecipitation of phospho-Jak3 and c-Met
Culture-generated B-lineage cells were washed, cytokine-starved for 5 hours, and stimulated with either rIL-7 (20 ng/mL) or rIL-7/HGFβ (60 ng/mL) for 10 to 30 minutes. The stimulated and unstimulated control cells were lysed in 25 mM Tris-HCL (pH 8), 150 mM NaCl, 1% Triton, 0.5% Igepal, and 1 mM sodium orthovanadate plus protease inhibitors. The supernatants were immunoprecipitated with antibodies against Jak3 or phosphotyrosine and protein G-agarose bead slurry. Pellets were resuspended in SDS sample buffer and subjected to Western blotting using antibodies against phosphotyrosine or phospho-c-Met.
Purification of the IL-7/HGFβ receptor
scIL-7/HGFβ (1 mg) was coupled to 0.2 mL cyanogen bromide (CNBr)-activated sepharose 4B according to the manufacturer's instructions. Purified CD19-AA4.1+ culture-generated cells (108; CLPs and pre-pro-B cells) were added to 2 mL lysis buffer (10 mM Tris-HCl buffer [pH 7.2],150 mM NaCl, and protease inhibitor cocktail in the presence of 1% Triton X-100), and the supernatant was added to a scIL-7/HGFβ affinity gel and gently rocked overnight at 4°C as described.31 After extensive washing with the lysis buffer (10 mM Tris-HCl buffer [pH 7.2] containing 0.15 M NaCl, 0.1% Triton X-100, and the protease inhibitor cocktail), the IL-7/HGFβ receptor was eluted in a stepwise fashion with 0.1 M glycine-HCL buffer (pH 3.3), 0.1 M glycine-HCl buffer (pH 2.0), and then 0.1 M sodium citrate buffer (pH 2.0) each containing 0.2M NaCl, 0.1% Triton X-100, and the protease inhibitor cocktail. The elutes were immediately neutralized with 2 M Tris base and subjected to SDS-PAGE and Western blotting.
Results
Expression and purification of a single-chain IL-7/HGFβ protein
Because the supernatants from the expressed insect cells had the highest thymocyte stimulatory activities (data not shown), this system was chosen for purification and biologic studies of the scIL-7/HGFβ protein. Inasmuch as the theoretical isoelectricpoint of the scIL-7/HGFβ is approximately 8, we reasoned that the protein should carry a positive charge at pH 7, thereby allowing it to flow through DEAE and to bind to CM resins. However, scIL-7/HGFβ proteins were eluted from both the DEAE and CM columns. Of importance, the protein that was eluted from the CM column was biologically active. This form of scIL-7/HGFβ was detected by the goat anti-IL-7 antibody from R&D Systems, but not by the rabbit anti-IL-7 antibody. In contrast, the scIL-7/HGFβ protein that was eluted from the DEAE column was biologically inactive and was detected by the rabbit, but not the goat, anti-IL-7. Furthermore, only the inactive form of IL-7/HGFβ reacted with the rabbit anti-HGFβ antibody, whereas both the active and inactive forms reacted with the goat anti-HGFβ. In addition, the active form of scIL-7/HGFβ had a molecular mass of 50 to 55 kDa, compared with 45 to 50 kDa for the inactive form (Figure S1; see the Supplemental Figures link at the top of the online article, at the Blood website). Inasmuch as the inactive form neither inhibited nor synergized with the active form of scIL-7/HGFβ (data not shown), only the properties of the active form are described.
Effect of scIL-7/HGFβ on early B-lineage development in vitro
The activity of the scIL-7/HGFβ protein on early B lymphocyte development was studied using freshly harvested rat and mouse BM cells. Consistent with our previous results,20,30 primary rat BM cell cultures generated pre-pro-B cells and pro-B cells in the presence of scIL-7/HGFβ, whereas cultures containing rIL-7 generated pro-B and pre-B cells almost exclusively (Figure S2).
As shown in Figure 2, mouse BM cells responded somewhat differently than did rat BM cells. Rather than containing B220+ cells only, approximately 60% of the mouse lymphoid cells in these cultures were B220-. Moreover, approximately two thirds of the B220- cells had a CD43+ HSA- phenotype, suggesting that some might be CLPs. To substantiate this, we used the protocol of Hardy and colleagues32 to demonstrate that approximately 30% of the total cells in the scIL-7/HGFβ-treated cultures had the AA4.1+ B220-HSA- CD4low phenotype characteristic of CLPs (Fraction Ao), 15% were early pre-pro-B cells (Fr. A1), 5% were late pre-pro-B cells (Fr. A2), and 20% were pro-B cells (Fr. B-C1). In contrast, Fr. Ao and A1 cells were absent from cultures containing rIL-7, and only 0.5% were A2 cells. Instead, 65% were pro-B cells (Fr. B-C), and 10% were pre-B cells (Fr. D). Cultures containing equimolar amounts both of IL-7 and scIL-7/HGFβ contained Fr. Ao through Fr. D cells.
When converted into mean numbers of cells per well (Table 2), the results showed an average overall 16-fold increase in output over input numbers of early B-lineage precursors in the scIL-7/HGFβ cultures, and a 27-fold increase in the rIL-7 cultures. However, the increases seen in the scIL-7/HGFβ cultures were restricted to the CLP (31-fold), pre-pro-B (8-fold), and pro-B (10-fold) cell fractions, whereas those in the IL-7 cultures involved the pro-B (51-fold) and the pre-B (> 32-fold) cell fractions only. Furthermore, the results in Figure 3A and 3B show that scIL-7/HGFβ selectively stimulated the proliferation of CLPs and pre-pro-B cells, and rIL-7 of pro-B cells and pre-B cells. Hence, the marked expansion of pro-B cells in the scIL-7/HGFβ cultures appears to be due primarily to their differentiation from proliferating pro-B cells.
Effect of scIL-7/HGFβ on CFU-S12
To determine if the scIL-7/HGFβ supported the survival or proliferation of more primitive lymphohemopoietic precursors then CLPs, irradiated mice were injected intravenously with culture-generated cells, and the number of macroscopically visible spleen colonies was determined 12 days later.33 Cells (1 × 106) from the scIL-7/HGFβ cultures generated 30 ± 4.2 CFU-S12 above background (minimal estimate due to partial confluency), as compared with a mean of only 1 colony for the IL-7 cultures. Histologic examination of the colonies generated by the scIL-7/HGFβ-cultured cells showed that many contained a mixture of erythroid, myeloid, and megakaryocytic elements. Furthermore, comparison with the CFU-S12 activity in freshly harvested BM showed that, on a per-cell basis, the scIL-7/HGFβ-stimulated cultures contained a normal frequency of CFU-S12. However, as the total number of cells in these cultures approximated that in the original inoculum (2 × 106), it was not possible to determine whether scIL-7/HGFβ maintained prolonged survival (> 15 days) of CFU-S12 or stimulated their (or their precursors') proliferation. Therefore, we repeated these experiments using cultures to which c-kitL (50 ng/mL) and flt-3L (50 ng/mL) had been added. Within 7 days, both the scIL/HGFβ and rIL-7 cultures contained approximately 5-fold more cells (8.8 × 106 to 12.8 × 106) than were present in the original inoculum. Again, only the scIL-7/HGFβ cultures contained significant CFU-S12 activity above background (c-kitL plus flt-3L only). However, as the total CFU-S12 activity per culture well exceeded the input activity by approximately 5-fold, it would appear the number of CFU-S12 in the scIL-7/HGFβ cultures had expanded by at least that amount.
Stimulation of mouse bone marrow cells by rIL-7 and/or scIL-7/HGFβ in vitro. Freshly harvested BM cells from IL-7-/- mice were cultured in RPMI-1640 containing 2-ME in the presence of 10 ng/mL rIL-7 or 30 ng/mL scIL-7/HGFβ, or both. Nonadherent cells were harvested at day 17 and analyzed by flow immunocytometry. Top row shows representative histograms of B220+ and B220- cells in each culture. The vertical standards indicate the peaks (or theoretical peak; dashed line) of florescence intensity and are used to eliminate most of the overlap regions between the peaks. Middle row shows the contour plots for CD43 and HSA of the B220- and B220+ cells to the left and right of the peaks in the top row. The various fractions of developing B-lineage cells and their relative proportions in the B220- and B220+ cell subsets are indicated for each quadrant. Bottom row shows the relative proportions of fractions Ao (CLPs), A1 (early pre-pro-B cells) and A2 (late pre-pro-B cells).
Stimulation of mouse bone marrow cells by rIL-7 and/or scIL-7/HGFβ in vitro. Freshly harvested BM cells from IL-7-/- mice were cultured in RPMI-1640 containing 2-ME in the presence of 10 ng/mL rIL-7 or 30 ng/mL scIL-7/HGFβ, or both. Nonadherent cells were harvested at day 17 and analyzed by flow immunocytometry. Top row shows representative histograms of B220+ and B220- cells in each culture. The vertical standards indicate the peaks (or theoretical peak; dashed line) of florescence intensity and are used to eliminate most of the overlap regions between the peaks. Middle row shows the contour plots for CD43 and HSA of the B220- and B220+ cells to the left and right of the peaks in the top row. The various fractions of developing B-lineage cells and their relative proportions in the B220- and B220+ cell subsets are indicated for each quadrant. Bottom row shows the relative proportions of fractions Ao (CLPs), A1 (early pre-pro-B cells) and A2 (late pre-pro-B cells).
Effect of scIL-7/HGFβ on early B-lineage development in vivo
To determine whether the culture-generated cells had functional lymphoid progenitor activity in vivo, 1 × 106 cells from cultures containing equimolar amounts of rIL-7 or scIL-7/HGFβ were injected intravenously into sublethally irradiated CD45-congenic mice. The results in Tables 3 and 4 show that, at 3 weeks, the cells from the scIL-7/HGFβ cultures generated 3- to 4-fold more splenic B-lineage cells than did those from the rIL-7 cultures, and the proportion of donor-origin B-lineage cells that had passed the pro-B cell stage greatly exceeded that in recipients of IL-7-cultured cells (P < .01). Furthermore, the B-cell generative activity of 1 × 106 cells from the scIL-7/HGFβ-containing cultures was equivalent quantitatively and qualitatively to that of a saturating dose (20 × 106) of normal BM cells, even though the latter contains a heterogeneity of cell types that might serve as lymphoid progenitors.
Incorporation of BrdU by culture-generated BM lymphoid cells stimulated or cross-stimulated in vitro with rIL-7 or scIL-7/HGFβ. BM cells from IL-7-/- mice were cultured in the presence of rIL-7 (10 ng/mL) or scIL-7/HGFβ (30 ng/mL) for 19 days. The cells were washed, cytokine-starved for 5 hours, stimulated with the homologous or heterologous cytokine for 3 hours, pulsed with BrdU, and stained with combinations of antibodies to B220, HSA, AA4.1, CD43, CD4, and BrdU. (A,C) Distribution early B-lineage subsets in each culture system. (B,D) Percentage of BrdU+ cells in each fraction of B-lineage cells. (A-B) ▪ indicates IL-7/HGFβ-generated cells stimulated with IL-7/HGFβ; ▦, IL-7-generated cells stimulated with IL-7. (C-D) ▪ indicates IL-7/HGFβ-generated cells stimulated with IL-7; ▦, IL-7-generated cells stimulated with IL-7/HGFβ. Means of duplicate samples are shown. Data are from 1 representative experiment of 2.
Incorporation of BrdU by culture-generated BM lymphoid cells stimulated or cross-stimulated in vitro with rIL-7 or scIL-7/HGFβ. BM cells from IL-7-/- mice were cultured in the presence of rIL-7 (10 ng/mL) or scIL-7/HGFβ (30 ng/mL) for 19 days. The cells were washed, cytokine-starved for 5 hours, stimulated with the homologous or heterologous cytokine for 3 hours, pulsed with BrdU, and stained with combinations of antibodies to B220, HSA, AA4.1, CD43, CD4, and BrdU. (A,C) Distribution early B-lineage subsets in each culture system. (B,D) Percentage of BrdU+ cells in each fraction of B-lineage cells. (A-B) ▪ indicates IL-7/HGFβ-generated cells stimulated with IL-7/HGFβ; ▦, IL-7-generated cells stimulated with IL-7. (C-D) ▪ indicates IL-7/HGFβ-generated cells stimulated with IL-7; ▦, IL-7-generated cells stimulated with IL-7/HGFβ. Means of duplicate samples are shown. Data are from 1 representative experiment of 2.
Identity of the receptor complex for scIL-7/HGFβ
Ordinarily, IL-7 binds to the α and γc chains of the IL-7R,34 whereas the α chain of mature HGF binds to the HGFR, c-Met.23 However, it is not known if the HGFβ chain also binds to c-Met. Therefore, to gain some insight into the nature of the receptor(s) for the scIL-7/HGFβ, antibodies specific for the IL-7Rα chain, the γc chain or c-Met were added to cultures of mouse early B-lineage cells generated in the presence of rIL-7 or scIL-7/HGFβ. As shown in Figure 4, the ability of scIL-7/HGFβ to stimulate the proliferation of BM lymphoid cells was partially, but significantly (P < .05), inhibited by antibodies to c-Met as well as those to the IL-7Rα and γc chains. Also, a mixture of anti-c-Met and anti-IL-7Rα antibodies showed greater inhibition than did either antibody alone. In contrast, only the antibodies to the IL-7Rα and γc chains inhibited proliferation stimulated by rIL-7.
Consistent with these blocking experiments, approximately 60% of the scIL-7/HGFβ culture-generated CLPs and pre-pro-B cells expressed both c-Met and the IL-7R, whereas more than 80% at the rIL-7 culture-generated pro-B cells expressed the IL-7R only (data not shown). Furthermore, confocal microscopy revealed that the IL-7 and c-Met receptors existed as aggregates that had undergone patching and capping on cells stimulated with scIL-7/HGFβ but not rIL-7 (Figure 5). Of interest, similar IL-7R/c-Met complexes were observed on enriched pre-pro-B cell fractions from normal (noncultured) BM, suggesting that they may have been stimulated by endogenous IL-7/HGFβ. In addition, cross-stimulation studies (Figure 3C-D) revealed that rIL-7 did not stimulate the proliferation of CLPs or pre-pro-B cells from scIL-7/HGFβ cultures, and that scIL-7/HGFβ did not stimulate pro-B cells or pre-B cells from rIL-7 cultures. However, as noted previously for native IL-7/HGFβ,30 scIL-7/HGFβ was able to “prime” pre-pro-B/pro-B cells to respond to rIL-7 (Figure 3D), presumably by up-regulating the IL-7Rα chain. Hence, in their aggregate, these results suggest that the scIL-7/HGFβ hybrid cytokine binds coordinately to the IL-7 and HGF receptors on B-cell precursors, whereas rIL-7 binds to the IL-7R only.
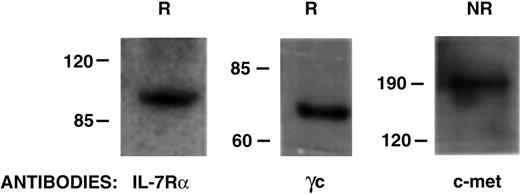
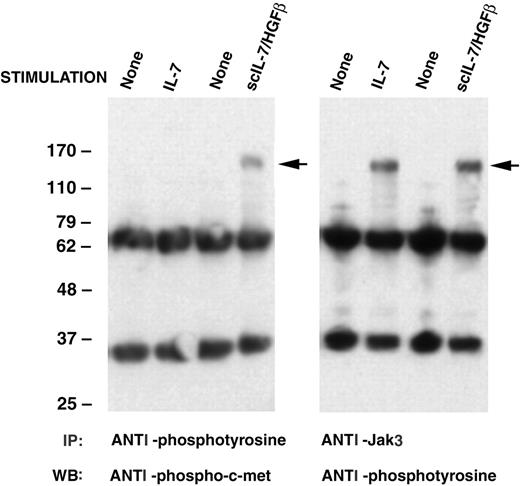
Direct evidence that both the IL-7R (α and γc chains) and c-Met are major components of the receptor complex for IL-7/HGFβ was provided on Western blots after purification of receptor proteins from culture-generated CLPs/pre-pro-B cells on a scIL-7/HGFβ affinity gel (Figure 6). In addition, demonstration that the binding of scIL-7/HGFβ to early B-lineage cells initiates signal transduction through both the IL-7R and c-Met was provided by analysis of phosphorylation of Jak3 (which associates with the γc chain of the IL-7R complex) and Western blotting with anti-c-Met phosphospecific antibody. Results in Figure 7 show that both rIL-7 and scIL-7/HGFβ transduce signals through the IL-7R, but that only scIL-7/HGFβ signals through c-Met as well.
Discussion
A novel recombinant IL-7/HGFβ hybrid cytokine was constructed as a single chain composed of IL-7 and HGFβ linked by a flexible, nonantigenic peptide (GGGGSGGGGS). After purifying the active form of scIL-7/HGFβ, we tested its activity in supporting B lymphogenesis in vitro. When rat BM cells were used, pre-pro-B cells and pro-B cells were selectively generated, as anticipated.21 However, when IL-7-/-mouse BM was used, scIL-7/HGFβ stimulated the proliferation of CLPs and early and late pre-pro-B cells, leading to the appearance of pro-B cells. Monomeric IL-7, on the other hand, primarily stimulated the proliferation and differentiation of pro-B cells and pre-B cells, even in the presence of SCF and/or flt-3L. In contrast, Kondo et al35 and Benz and Bleul36 have reported that IL-7 can stimulate the clonal expansion of CLPs when mixed with SCF and flt3L. It should be noted, however, that their experiments were conducted under semisolid, rather than liquid, culture conditions. Similarly, even in laboratories using liquid cultures (± stromal cell layers), some have reported that rIL-7 stimulates CLPs,4-7 and some that it does not.8-14,37 Furthermore, in a recent report of IL-7-induced CLP differentiation,38 proliferation of these cells was not observed. An explanation for these differences may reside in the observation of Dias et al39 that signaling through the IL-7R is required to maintain the B-lineage developmental potential of CLPs. Therefore, we can only presume that the IL-7/HGFβ hybrid cytokine fulfils this potential while unequivocally stimulating the proliferation of CLPs.
Ability of antibodies to the IL-7R and/or c-Met to inhibit the stimulation of mouse bone marrow cells by rIL-7 or scIL-7/HGFβ. Culture-generated BM cells (4 × 105 cells/well) from IL-7-/- mice were incubated for 3 days in the presence of rIL-7 (10 μg/mL) or scIL-7/HGFβ (30 μg/mL) to which antibodies against IL-7Rα, γc, and/or c-Met (10 μg/mL) were added. Incorporation of [methyl-3H] thymidine (mean counts per minute [CPM] ± SD) was determined after a 12-hour pulse. *P < .05 between antibody-treated and untreated values (similar results were obtained with isotype controls). **P < .05 versus value for anti-IL-7Rα or anti-HGFR alone. One representative experiment of 4 is shown.
Ability of antibodies to the IL-7R and/or c-Met to inhibit the stimulation of mouse bone marrow cells by rIL-7 or scIL-7/HGFβ. Culture-generated BM cells (4 × 105 cells/well) from IL-7-/- mice were incubated for 3 days in the presence of rIL-7 (10 μg/mL) or scIL-7/HGFβ (30 μg/mL) to which antibodies against IL-7Rα, γc, and/or c-Met (10 μg/mL) were added. Incorporation of [methyl-3H] thymidine (mean counts per minute [CPM] ± SD) was determined after a 12-hour pulse. *P < .05 between antibody-treated and untreated values (similar results were obtained with isotype controls). **P < .05 versus value for anti-IL-7Rα or anti-HGFR alone. One representative experiment of 4 is shown.
Expression of the IL-7R and/or c-Met on culture-generated and normal pre-pro-B cells and pro-B cells. Images of IL-7R (green) and c-Met (red) examined by confocal microscopy show colocalization (yellow) patching and capping of IL-7R and c-Met on scIL-7/HGFβ culture-generated and enriched pre-pro-B cells from normal BM. IL-7 culture-generated cells expressed the IL-7R only. Some cytoplasmic staining is also seen in the latter cells, which were stained after the smears had been fixed. The top panels are isotype controls. Scale bars equal 10 μm.
Expression of the IL-7R and/or c-Met on culture-generated and normal pre-pro-B cells and pro-B cells. Images of IL-7R (green) and c-Met (red) examined by confocal microscopy show colocalization (yellow) patching and capping of IL-7R and c-Met on scIL-7/HGFβ culture-generated and enriched pre-pro-B cells from normal BM. IL-7 culture-generated cells expressed the IL-7R only. Some cytoplasmic staining is also seen in the latter cells, which were stained after the smears had been fixed. The top panels are isotype controls. Scale bars equal 10 μm.
Analysis of the purified IL-7/HGFβ receptor proteins. The IL-7/HGFβ receptor complex was isolated on a scIL-7/HGFβ affinity gel from purified culture-generated CLP/pre-pro-B cells. The eluates were subjected to SDS-PAGE under reducing (R) or nonreducing (NR) conditions, and Western blotting was done with antibodies to IL-7Rα, γc, or c-Met.
Analysis of the purified IL-7/HGFβ receptor proteins. The IL-7/HGFβ receptor complex was isolated on a scIL-7/HGFβ affinity gel from purified culture-generated CLP/pre-pro-B cells. The eluates were subjected to SDS-PAGE under reducing (R) or nonreducing (NR) conditions, and Western blotting was done with antibodies to IL-7Rα, γc, or c-Met.
Ability of rIL-7 or scIL-7/HGFβ to activate Jak3 and/or c-Met in mouse B-lineage bone marrow cells. B-lineage cells generated in cultures of IL-7-/- mouse BM cells supplemented with rIL-7 or scIL-7/HGFβ were harvested, placed in cytokine-free medium for 5 hours, and then stimulated with the homologous cytokine for 10 or 30 minutes. The supernatants from lysed cells were immunoprecipitated with anti-Jak3 or antiphosphotyrosine antibody and subjected to SDS-PAGE and Western blotting using the indicated antibodies. Arrows indicate phospho-c-Met or phospho-Jak3.
Ability of rIL-7 or scIL-7/HGFβ to activate Jak3 and/or c-Met in mouse B-lineage bone marrow cells. B-lineage cells generated in cultures of IL-7-/- mouse BM cells supplemented with rIL-7 or scIL-7/HGFβ were harvested, placed in cytokine-free medium for 5 hours, and then stimulated with the homologous cytokine for 10 or 30 minutes. The supernatants from lysed cells were immunoprecipitated with anti-Jak3 or antiphosphotyrosine antibody and subjected to SDS-PAGE and Western blotting using the indicated antibodies. Arrows indicate phospho-c-Met or phospho-Jak3.
Our observations that the IL-7/HGFβ hybrid cytokine binds to and signals through both the IL-7 and c-Met receptors, and that these receptors form complexes on the surface of CLPs and pre-pro-B cells, indicate that the IL-7Rα chain, the γc chain, and c-Met are key components of the receptor complex for IL-7/HGFβ. However, it must be cautioned that additional, as-yet-unidentified components may be required to form a high affinity IL-7/HGFβR. For example, both IL-7 and mature HGF use heparan-sulfated proteoglycans (HSPGs) as low-affinity receptors to optimize presentation of ligand to their respective receptors;23,40 and certain CD44 variant (v) isoforms bearing heparan sulfate (HS) side-chains not only facilitate the presentation of HGF to c-Met, but assist in signal transduction by associating with the cytoplasmic tail of c-Met.41 In addition, after being secreted by the BM stromal cells, IL-7, HGF and the free HGFβ chain bind to HSPGs on the stromal cell surface and in the extracellular matrix,6,9,19,22-24,27,42-44 where the HSPGs presumably assist in the assembly of the IL-7/HGFβ hybrid cytokine.21 These considerations may help to explain the contact dependency of CLPs and pre-pro-B cells.
Nonetheless, as IL-7/HGFβ can maintain the viability of pro-B cells, and IL-7 of CLPs and pre-pro-B cells, it would appear that both can bind to (and signal through) the IL-7R on all of these cells. However, as the IL-7R transmits distinct signals for proliferation and differentiation,45 the present results suggest that IL-7/HGFβ cannot induce a proliferative signal through the IL-7R alone, and that IL-7 cannot do so in the presence of IL-7R/c-Met complexes and/or low levels of IL-7Rα. Furthermore, IL-7 itself may require pre-B cell receptor expression or interaction with other cytokines to transduce a proliferative signal on pro-B cells.9,11,12,14,46 These considerations plus the apparently selective expression of c-Met by CLPs and pre-pro-B cells, but few pro-B cells, may provide an explanation for the sequential and stage-specific activities of IL-7/HGFβ and IL-7 on early B-lineage development. This notion is supported by the observations that IL-7/HGFβ up-regulates the IL-7Rα chain on early pro-B cells,30 and HGFβ is the mitogenic component of mature HGF.47 Our view, therefore, is that the IL-7/HGFβ hybrid cytokine is the preferred ligand for stimulating CLPs and pre-pro-B cells in mouse BM, and IL-7 for stimulating pro-B cells and pre-B cells.
The downstream activation events that follow signaling with IL-7/HGFβ are unknown. Ordinarily, IL-7-induced signaling primarily involves a number of tyrosine kinase pathways including Jak/STAT, Src, and p13-K,48 whereas HGF signaling involves the Grab2/RAS/MAPK and Gab1/PI3K/AKt pathways.23 However, the signaling pathways (and the functional readouts) that are activated by coordinate (juxtacrine) signaling through the IL-7R/c-Met complex may differ quantitatively and/or qualitatively from those activated by IL-7 or HGF alone. Thymic stromal lymphopoietin (TSLP) may be instructive in the regard, as it interacts both with the IL-7Rα chain and the TSLP receptor to generate a signal that is dramatically different from that generated by IL-7.49 Similarly, IL-23 transmits unique signals through a receptor composed of the IL-12Rβ1 chain and the IL-23R.50 Additionally, ligand-induced heterodimers of CCR2 and CCR5 signal at 10- to 100-fold lower concentrations of MCP-1 and RANTES than required for either chemokine alone, and they stimulate cell adhesion rather than chemotaxis.51 Of even greater relevance, mature HGF has been shown to synergize with a number of cytokines and growth factors during lymphohemopoiesis (eg, IL-3, IL-11, granulocyte-macrophage colony-stimulating factor [GM-CSF], erythropoietin [EPO]) and, in the case of SCF, to induce juxtacrine interactions between c-kit and c-Met that result in the support of multipotent colony formation.52,53 Thus, the IL-7/HGFβ complex would appear to be designed to ensure that similar juxtacrine interactions occur between the IL-7R and c-Met.
In adoptive transfer studies, BM cell cultures containing scIL-7/HGFβ were 3 to 4 times more efficient at generating peripheral B cells in vivo than were cultures containing equimolar amounts of rIL-7. In addition, the ratios of immature (CD43+) to more mature (CD43-) B-lineage cells in the BM of recipients of scIL-7/HGFβ-cultured cells more closely approximated those generated by normal BM cells than by IL-7-cultured cells. These observations suggest that differences exist between both the nature and number of the B-lineage progenitors produced under these 2 culture conditions. It must be emphasized that the adoptive hosts in these experiments received only 1 × 106 culture-generated BM cells, as compared with a saturating dose of 20 × 106 normal BM cells. Hence, normalization of the data in Table 3 reveals that, on a per-cell basis, the BM cells from rIL-7/HGFβ cultures were approximately 16-fold and 20-fold more active, respectively, than were normal BM cells at generating B-lineage cells in the BM and spleen. However, due to the greater heterogeneity of cell types in the whole versus the cultured BM cell suspensions, it remains to be determined whether the same precursor cell populations are being compared. It also remains to be determined whether the BM cells from rIL-7 and scIL-7/HGFβ-containing cultures differ in their abilities to generate thymocytes and T cells in vivo. This seems possible, given that the ability of rIL-7 and IL-7/HGFβ to stimulate thymocyte proliferation in vitro20 differs in terms of receptor requirements (L.L., R.A.Z., and I.G., unpublished observation, April 2004).
Another major difference in the respective stimulatory properties of scIL-7/HGFβ and IL-7 is the ability of the scIL-7/HGFβ to support the growth of CFU-S12 in vitro. Whether or not some of these CFU-S12 are true hematopoietic stem cells (HSCs) remains to be determined,54 especially as HGF has itself been reported to affect the proliferation and differentiation of hemopoietic stem and progenitor cells.23,24,27,43,55 The mechanism by which scIL-7/HGFβ stimulates CFU-S12 in cultures of mouse BM cells is unknown, as these cells and their precursors do not appear to express the IL-7Rα chain (Adolfsson et al56 and L.L., R.A.Z., and I.G., unpublished observations May 2005). One possibility is that the HGFβ chain of the IL-7/HGFβ hybrid cytokine may stimulate CFU-S12 through c-Met, possibly in concert with binding of the ubiquitous γc chain. Indeed, we have observed (L.L., R.A.Z., and I.G., unpublished observation, December 2004) that an equimolar concentration of mature HGF also maintains CFU-S12 activity in mouse BM cultures. Another possibility is that scIL-7/HGFβ affects CFU-S12 proliferation indirectly by activating a separate population of IL-7R+ c-Met+ cells. Experiments are in progress to determine whether other cytokines known to influence early B-lineage development can synergize with scIL-7/HGFβ to enhance the generation of HSCs, CFU-S12, and CLPs in vitro. The in vivo correlates of these experiments are also being pursued to determine if scIL-7HGFβ can enhance BM engraftment and/or restore lymphopoiesis in mice with primary or secondary immunodeficiencies.
Prepublished online as Blood First Edition Paper, November 22, 2005; DOI 10.1182/blood-2005-08-3470.
Supported in part by National Institutes of Health Grant AI32752.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Figure 4. Ability of antibodies to the IL-7R and/or c-Met to inhibit the stimulation of mouse bone marrow cells by rIL-7 or scIL-7/HGFβ. Culture-generated BM cells (4 × 105 cells/well) from IL-7-/- mice were incubated for 3 days in the presence of rIL-7 (10 μg/mL) or scIL-7/HGFβ (30 μg/mL) to which antibodies against IL-7Rα, γc, and/or c-Met (10 μg/mL) were added. Incorporation of [methyl-3H] thymidine (mean counts per minute [CPM] ± SD) was determined after a 12-hour pulse. *P < .05 between antibody-treated and untreated values (similar results were obtained with isotype controls). **P < .05 versus value for anti-IL-7Rα or anti-HGFR alone. One representative experiment of 4 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-08-3470/2/m_zh80050692160004.jpeg?Expires=1769535450&Signature=jSp5Xd1ksKhJv7CgiypPg-hQvgBkL6ZKZT3WJbM1wLuWm0S6pDoHT9h4Gd8Z4NcL6T~OiKXej72IeKRX1inqIejZDHti~yvAzyOmrJpM1LGnMT7x519xI5AjX8ePlkbv~YHfw3qLTy7ssgvxsEDZ5ImuKEojRZxZCrZNPImt8N4gAW6WZY7sMmyia9Mopm1sq0OtCC5r~SVmQqXFUYasfKfgjSDBtOhLBepV9k~PZ-E0YcVkHLRVVnlhybgf2lINjk2Rc~ur9NZ6clSvZXZSGdCPXyeeWylyht20IdNI~mjWeT7dvDNsbjFGK3107~XNvR1ohZKDBac8QzlllahvwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)