Neutrophil migration requires continuous reorganization of the cytoskeleton and cellular adhesion apparatus. Chemoattractants initiate intracellular signals that direct this reorganization. The signaling pathways that link chemoattractant receptors to the cytoskeleton and cellular adhesion apparatus are now being defined. Formyl-peptide chemoattractants released from bacteria stimulate G-protein–linked receptors on the surface of neutrophils and regulate the neutrophil cytoskeleton and adhesion apparatus through RhoA-dependent pathways. Lsc is a RhoA guanine nucleotide exchange factor that binds the heterotrimeric G-protein α-subunits, Gα12 and Gα13. We have disrupted the Lsc gene and demonstrated that formyl-peptide–stimulated Lsc knock-out (KO) neutrophils are unable to generate and sustain a single-dominant pseudopod and migrate with increased speed and reduced directionality. Unexpectedly, we also found that Lsc is required for normal β2- and β1-integrin–dependent neutrophil adhesion. Lsc-deficient mice have a peripheral leukocytosis and extramedullary hematopoiesis, demonstrating that Lsc is required for leukocyte homeostasis. Lsc-deficient neutrophils are recruited normally to sites of bacterial peritonitis and chemical dermatitis, indicating that other signaling pathways compensate for the Lsc deficiency in some forms of inflammation. These results demonstrate that Lsc links formyl-peptide receptors to RhoA signaling pathways that regulate polarization, migration, and adhesion in neutrophils and that Lsc is required for leukocyte homeostasis.
Introduction
Neutrophils migrate from the peripheral blood to extravascular sites of action in response to chemoattractants released by pathogens and host cells. This directional migration requires continuous reorganization of the neutrophil's cytoskeleton and adhesion apparatus.1 The intracellular signaling pathways that link chemoattractant receptors to the cytoskeleton and adhesion apparatus are now being defined. These pathways are attractive targets for pharmacologic manipulation of neutrophils, because migration is central to neutrophil function.
Bacterial proteins are translated with an N-terminal formylated methionine, and the resulting formyl-peptides are potent neutrophil chemoattractants. They stimulate G-protein–linked formyl-peptide receptors, FPR and FPRL1,2 and activate downstream RhoA-dependent signaling pathways that regulate neutrophil cytoskeletal reorganization3 and adhesion.4-6 Understanding how formyl-peptide receptors couple to RhoA will provide insight into how other chemoattractants that stimulate G-protein–linked receptors, including complement fragments, leukotriene B4, and interleukin 8, control neutrophil migration.
Ligands for G-protein–linked receptors can activate RhoA through associated heterotrimeric G-protein α-subunits from the Gα12 (Gα12 and Gα13)7-9 and Gαq10,11 families. Recently, Gα12 and Gα13 were implicated in formyl-peptide–stimulated cytoskeletal reorganization in neutrophils. Differentiated myeloid HL-60 cells are a commonly used model for neutrophil migration, because they have similar formyl-peptide–stimulated polarization and chemotactic responses as neutrophils.12 Inhibition of Gα12, Gα13, or RhoA in formyl-peptide–stimulated differentiated HL-60 cells induces super-numerary pseudopodia that form around the entire perimeter of the cell; they are unable to form a single-dominant pseudopod.3 In complementary experiments, formyl-peptide–stimulated differentiated HL-60 cells expressing constitutively activated Gα12, Gα13, or RhoA do not form a mature pseudopod.3 Together, these results suggest that Gα12 and Gα13 activate RhoA signaling pathways that direct the formation of a single-dominant pseudopod in formyl-peptide–stimulated neutrophils.
Rho GTPases cycle between a GTP-bound active form and a GDP-bound inactive form. Rho guanine nucleotide exchange factors (GEFs) activate Rho GTPases by promoting the release of GDP in exchange for GTP. The large family of Dbl-Rho GEFs (> 65 human genes) is characterized by 2 conserved domains: a catalytic Dbl homology (DH) domain that facilitates GDP release and a pleckstrin homology (PH) domain that directs the GEF's intracellular location and/or regulates the DH domain.13,14 There is a small subfamily of at least 4 RhoA GEFs that link G-protein α-subunits to RhoA: Lsc/p115 RhoGEF,15-19 leukemia-associated Rho GEF (LARG),20,21 PDZ-RhoGEF/GTRAP48,22 and Lbc23 share an N-terminal RGS (regulator of G-protein signaling) domain that binds Gα12 and Gα13. LARG can also bind Gαq.20,21 Lsc is a 919 amino acid (aa) RhoA GEF that is heavily expressed in hematopoietic tissue.15-19 The C-terminus of Lsc contains residues that inhibit Lsc function and a coiled-coiled domain that is required for Lsc homo-oligomerization.24 Gα12 and Gα13 can stimulate the DH domain of Lsc to catalyze RhoA activation,19 whereas the RGS domain of Lsc can inhibit Gα12 and Gα13.18,25 Lsc has been genetically implicated in marginal zone B (MZB) cell migration. The murine Lsc locus has been disrupted, and Lsc-deficient MZB cells have increased chemokinesis and chemotaxis compared with fetal calf serum and have increased chemokinesis compared with mouse serum.26
We hypothesized that Lsc couples formyl-peptide G-protein–linked receptors to the RhoA signaling pathways that regulate pseudopod formation in neutrophils. Here, we have disrupted the Lsc locus by homologous recombination and generated Lsc-/- mice. We have used neutrophils from these mice to demonstrate that Lsc is required for normal polarization, migration, and adhesion of formyl-peptide–stimulated neutrophils. Lsc-deficient mice have a peripheral leukocytosis with splenomegaly and extramedullary hematopoiesis, demonstrating that Lsc is also required for normal leukocyte homeostasis in vivo.
Materials and methods
Antibodies
M-19 goat anti-Lsc, C-23 rabbit-Sos1, 119 rabbit anti-RhoA, C-11 rabbit anti-Rac2, and HRP-conjugated rabbit antigoat antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa 594–conjugated phalloidin, Alexa 488–conjugated donkey anti–goat IgG, Alexa 488–conjugated goat anti–rabbit IgG, Alexa 488–conjugated goat anti–mouse IgM, and Alexa 488–conjugated goat anti–mouse IgG antibodies were obtained from Molecular Probes (Eugene, OR). Mouse M2 anti-FLAG antibody was obtained from Sigma (St Louis, MO). Mouse anti-Rho A, B, C; mouse anti-Cdc42; and mouse anti-Rac1 antibodies were obtained from Pierce (Rockford, IL). Mouse monoclonal IgM anti–phosphatidylinositol-3,4,5-triphosphate antibody was obtained from Echelon Biosciences (Salt Lake City, UT). Rabbit anti–phospho-Akt (Ser473) and anti–phosphomyosin light chain 2 (Ser19) antibodies were obtained from Cell Signaling Technology (Beverly, MA). HRP-conjugated goat anti–mouse IgG antibody was obtained from Bio-Rad (Hercules, CA). FITC-conjugated anti–Gr-1 (RB6-8C5), PE-conjugated anti-CD18 (C71/16), FITC-conjugated anti-CD29 (Ha2/5), FITC-conjugated isotype control (A95-1), PE-conjugated isotype control (R35-95), FITC-conjugated isotype control (G235-1), and anti–Fc receptor antibodies were obtained from Becton Dickinson (Franklin Lakes, NJ).
Isolation of mouse bone marrow neutrophils
Mouse bone marrow neutrophils were isolated using a modification of a previously published protocol.27 Bone marrow was flushed from isolated mouse femurs and tibias into HBSS (without Ca2+ or Mg2+) supplemented with 1 mM HEPES, 0.5% BSA, and 1% dextrose, the cells were collected by centrifugation at 1100g for 5 minutes at 4°C, and erythrocytes were lysed in hypotonic lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA) for 4 minutes at room temperature. The suspension was passed through a 70-μm strainer (Becton Dickinson); cells were collected by centrifugation at 1100g for 5 minutes at 4°C, washed, resuspended in 45% wt/vol Percoll (Amersham Biosciences, Piscataway, NJ) in HBSS (without Ca2+ or Mg2+) supplemented with 1 mM HEPES, layered onto a discontinuous 62%/80% Percoll gradient prepared in HBSS (without Ca2+ or Mg2+), and centrifuged in the Percoll gradient at 2700g for 30 minutes at 4°C. The cells at the 62%/80% interface were retrieved, washed, and resuspended in migration buffer for use in experiments. In some experiments, this protocol was modified to include only the 62% Percoll layer, the Percoll centrifugation was at 1100g, and cells were isolated below this layer as described.28 Wright-Giemsa staining and fluorescence-activated cell sorting (FACS) analysis after labeling the cells with PE-conjugated anti–Gr-1 antibody revealed that approximately 90% of the resuspended cells isolated with these methods are neutrophils.
Immunostaining bone marrow neutrophils
Bone marrow neutrophils were isolated, allowed to adhere to a glass coverslip for 30 minutes, incubated with vehicle (DMSO) or fMLP for 5 minutes, fixed in 4% paraformaldehyde (PFA), permeabilized with 0.5% Triton, and then labeled with Alexa 594–conjugated phalloidin and the indicated antibodies.
Animal care
Mouse care was provided in accordance with Weill Medical College of Cornell University Institutional Animal Care and Use Committee (IACUC) Protocol 0202-938A.
Targeted disruption of Lsc
Lsc was disrupted in RF8 129/SvJae murine embryonic stem (ES) cells by homologous recombination with a targeting vector constructed by inserting a 1926 nucleotide (nt) upstream arm and a 4212 nt downstream arm derived from a 129/SvJ genomic bacterial artificial chromosome (clone no. 480(f8); Genome Systems, St Louis, MO) into the pNTK-loxP vector.29 Integration of the targeting vector replaces 1370 nt upstream of the start codon, the start codon, and the first 13 translated exons of Lsc, encoding the N-terminal 420 aa of the protein. Homologous recombination of the targeting vector in the ES cells was confirmed by Southern blot with probes outside the 5′ and 3′ boundaries of the vector. Three correctly targeted ES-cell clones were identified. Targeted ES cells were aggregated with C57BL/6 blastocysts and implanted into C57BL/6 females to generate chimeric progeny. The chimeric progeny were bred to C57BL/6 mice to generate 50:50 129/SvJae: C57BL/6 progeny that were interbred to generate the 12- to 20-week-old mice used for all experiments.
Zigmond chamber time-lapse microscopy
Bone marrow neutrophils were isolated, resuspended in migration buffer (HBSS with Ca2+ and Mg2+ supplemented with 0.2% BSA and 1 mM HEPES) at 2 to 4 × 106 cells/mL, allowed to adhere to a glass coverslip for 15 minutes, placed in a Zigmond chamber (Neuroprobe, Gaithersburg, MD) loaded with 10 μM fMLP in migration buffer supplemented with 1% gelatin in both reservoirs, and equilibrated for 10 minutes; the chamber was placed in a stage heater at 37°C for imaging. Wide-field DIC images of cells on the chamber bridge were acquired with an Axiovert 200M inverted microscope (Zeiss, Jena, Germany) equipped with 100 W halogen light source, using an oil-immersion 25 × /0.80 numerical aperture plan Neofluar/DIC objective (Zeiss). Images were acquired at 15-second intervals for 15 minutes using a 70-millisecond exposure time and recorded with a Micromax CCD camera (Princeton Instruments, Trenton, NJ) using Metamorph software (Universal Image, West Chester, PA).
Quantitative analysis of neutrophil morphology and migration
Image analysis was conducted with Metamorph software (Universal Image, West Chester, PA). The centroid was marked, and the perimeter was traced for all cells demonstrating any movement (> 80% of cells) in a standardized region (central 75% of field) of each of the 61 images acquired in each 15-minute experiment. The following definitions were used: area (μm2) was the area enclosed by the traced perimeter of the cell30 ; roundness (%) was 100 × 4π × area/(perimeter)2,30 ; total linear distance traveled (μm) was the sum of the distances between a cell's centroid in successive images over 15 minutes; net distance traveled (μm) was the linear distance between a cell's centroid in the first and last images from a 15-minute experiment; speed (μm/minute) was the total linear distance traveled in 15 minutes/15 minutes; direction change (degrees/turn) was the absolute value of the angle between the line joining the cell's centroid in the current and subsequent frame and the line joining the cell's centroid in the current and previous frame.30 Angles with an absolute value more than 180° were subtracted from 360° so that all angles are less than 180°; directionality (μm/μm) was the net distance traveled/total linear distance traveled.31,32 (+) or (-) Δ area, Δ perimeter, Δ roundness were the sum of all increases (+) or sum of all decreases (-) in area, perimeter, or roundness, respectively, between successive frames over 15 minutes. Mean ± SEM values were derived from all scored cells from 2 fields in each of 3 independent experiments (n = 60 cells for each genotype).
Neutrophil adhesion assay
Bone marrow neutrophils were isolated, resuspended at 106 cells/mL in adhesion buffer (HBSS with Ca2+, Mg2+, and 20 mM HEPES); 105 cells/well were placed in a 96-well Microlite 2+ flat-bottom plate (no. 7572; Thermo Labsystems, Franklin, MA) and allowed to adhere for 60 minutes. The cells were stimulated with 10 μM fMLP for 30 minutes and washed 3 times with 100 μL PBS, 50 μL fresh adhesion buffer was added to each well, and the number of adherent cells was measured using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI) with a MicroLumat Plus LB96V 96-well luminometer (Berthold Technologies, Bad Wildbad, Germany). Adherent fraction of plated cells was the luminescent signal of washed cells normalized to the mean luminescent signal for 105 cells of that genotype. The surface of the microtiter plate wells was coated with fibronectin by placing 50 μL fibronectin in PBS (20 μg/mL) in the well for 2 to 3 hours at room temperature.
Statistics
All comparisons were made using a 2-tailed, unpaired, Student t test, except where indicated.
Online description of methods
The following methods are explained in the Supplemental Materials (available at the Blood website; click on the Supplemental Materials link at the top of the online article): Southern blot of genomic tail DNA, isolation of mouse splenocytes, reverse transcriptase–polymerase chain reaction (RT-PCR) amplification of Lsc partial cDNAs from splenocyte RNA, Western blotting, activated Rho GTPase pull-down assays, integrin expression, complete peripheral-blood counts, Escherichia coli peritonitis, and chemical dermatitis.
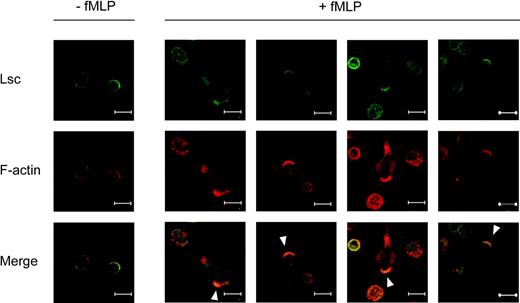
Lsc is specifically enriched at the leading and trailing edges of fMLP-stimulated neutrophils. Confocal images of murine bone marrow neutrophils adhered to a glass coverslip and incubated with vehicle alone (-fMLP) or with 1 μM fMLP (+fMLP) for 5 minutes. The cells were then fixed, permeabilized, and labeled with anti-Lsc antibody (green) and phalloidin (red). The majority of Lsc is symmetrically distributed at the plasma membrane of unstimulated neutrophils. Lsc becomes enriched at the leading edge (solid arrowhead) and, to a lesser extent, the trailing edge of neutrophils stimulated with fMLP. Representative of 2 independent experiments. Bars represent 10 μm.
Lsc is specifically enriched at the leading and trailing edges of fMLP-stimulated neutrophils. Confocal images of murine bone marrow neutrophils adhered to a glass coverslip and incubated with vehicle alone (-fMLP) or with 1 μM fMLP (+fMLP) for 5 minutes. The cells were then fixed, permeabilized, and labeled with anti-Lsc antibody (green) and phalloidin (red). The majority of Lsc is symmetrically distributed at the plasma membrane of unstimulated neutrophils. Lsc becomes enriched at the leading edge (solid arrowhead) and, to a lesser extent, the trailing edge of neutrophils stimulated with fMLP. Representative of 2 independent experiments. Bars represent 10 μm.
Results
Lsc is enriched at the leading and trailing edges of formyl-peptide–stimulated neutrophils
We used confocal microscopy to demonstrate that Lsc is expressed in murine bone marrow neutrophils and then to define its intracellular distribution before and after stimulation with the formyl-peptide chemoattractant, N-formyl-methionyl-leucyl-phenylalanine (fMLP). Lsc is symmetrically distributed at the plasma membrane of unstimulated neutrophils (Figure 1). Within 5 minutes of fMLP stimulation, the neutrophils assume a polarized morphology, and Lsc becomes enriched at the leading edge of the pseudopod and, to a lesser extent, at the trailing edge of the uropod (Figure 1). Thus, Lsc is enriched at the sites of active cytoskeletal reorganization in fMLP-stimulated neutrophils. The distribution of Lsc in a second myeloid-cell type, HL-60 cells, is nearly identical to that in neutrophils before and after formyl-peptide stimulation (Supplemental Figure S1). Sos1 is another Dbl-Rho GEF with a PH domain. Unlike Lsc, it is distributed diffusely in the cytoplasm of unstimulated neutrophils and is not enriched in the pseudopod or uropod of neutrophils stimulated with fMLP (Supplemental Figure S2). This demonstrates that the polarized distribution of Lsc in neutrophils is specific and not simply due to the presence of a PH domain.
Targeted disruption of Lsc
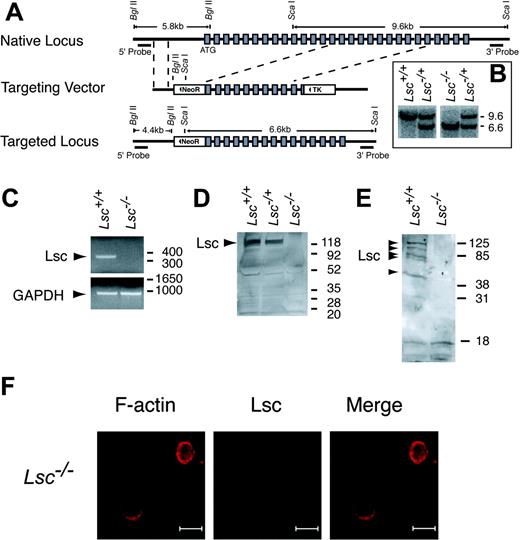
To determine the function of Lsc in neutrophils, we disrupted the Lsc gene to generate Lsc-/- (Lsc KO) mice (Figure 2A-F). Lsc KO mice do not produce Lsc (Figure 2D-E), are viable at birth, appear normal, and have a normal life span. Lsc-/+ matings produce offspring carrying the targeted allele at the expected Mendelian frequencies (Lsc+/+, 68 mice; Lsc-/+, 143 mice; Lsc-/-, 69 mice), indicating that Lsc is not required for embryonic development in the 50:50 C57BL/6:129/SVJae genetic background.
Targeted disruption of the Lsc gene eliminates Lsc expression. (A) Schematic diagrams of the native Lsc gene locus, Lsc targeting vector, and targeted Lsc gene locus. Shaded boxes indicate translated exons; dashed lines, boundaries of recombination between the native locus and targeting vector; solid triangles, orientation of the neomycin resistance (NeoR) and the thymidine kinase (TK) genes. (B) Southern blot of tail genomic DNA from the progeny of an Lsc-/+ mating, with the 3′ probe that hybridizes to a 9.6-kilobase (kb) fragment released from the native locus and a 6.6-kb fragment released from the targeted locus. Size markers at right are kb. (C) RT-PCR products generated from splenocyte oligo dT-primed total RNA with primers for Lsc and GAPDH. Size markers at right are base pair (bp). (D-E). Western blots of splenocyte (D) and neutrophil (E) lysates with anti-Lsc antibody. Multiple bands in Lsc+/+ neutrophil lysates may be Lsc splice variants or degradation products. Molecular weight markers at right, kDa. (F) Confocal images of Lsc KO neutrophils prepared and labeled as described in Figure 1. Bars represent 10 μm.
Targeted disruption of the Lsc gene eliminates Lsc expression. (A) Schematic diagrams of the native Lsc gene locus, Lsc targeting vector, and targeted Lsc gene locus. Shaded boxes indicate translated exons; dashed lines, boundaries of recombination between the native locus and targeting vector; solid triangles, orientation of the neomycin resistance (NeoR) and the thymidine kinase (TK) genes. (B) Southern blot of tail genomic DNA from the progeny of an Lsc-/+ mating, with the 3′ probe that hybridizes to a 9.6-kilobase (kb) fragment released from the native locus and a 6.6-kb fragment released from the targeted locus. Size markers at right are kb. (C) RT-PCR products generated from splenocyte oligo dT-primed total RNA with primers for Lsc and GAPDH. Size markers at right are base pair (bp). (D-E). Western blots of splenocyte (D) and neutrophil (E) lysates with anti-Lsc antibody. Multiple bands in Lsc+/+ neutrophil lysates may be Lsc splice variants or degradation products. Molecular weight markers at right, kDa. (F) Confocal images of Lsc KO neutrophils prepared and labeled as described in Figure 1. Bars represent 10 μm.
Lsc is required for normal pseudopod formation in formyl-peptide–stimulated neutrophils
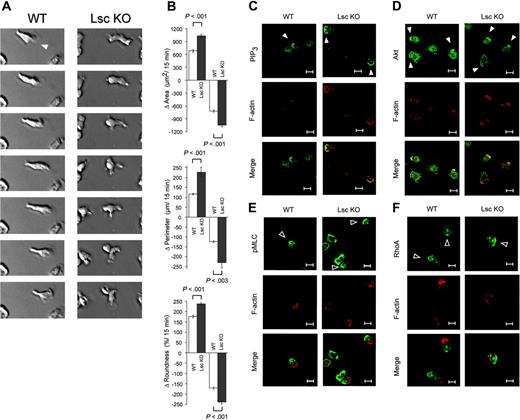
We compared the morphology of WT and Lsc KO neutrophils stimulated with fMLP. Differential interference contrast (DIC) photomicrographs of WT and Lsc KO neutrophils stimulated with a uniform concentration of fMLP were collected at 15-second intervals for 15 minutes. WT neutrophils stimulated with fMLP generate and sustain a single-dominant pseudopod, just prior to and during migration (Figure 3A; Supplemental Video S1). In contrast, Lsc KO neutrophils rapidly generate and retract pseudopodia at inappropriate locations around the entire perimeter of the cell (Figure 3A; Supplemental Video S2). As a result, Lsc KO neutrophils are unable to form and sustain a single-dominant pseudopod and often maintain supernumerary pseudopodia concurrently. Quantitative analyses confirmed the magnitude and significance of these differences. Lsc KO neutrophils have markedly larger cumulative interval increases and decreases in area, perimeter, and roundness during the 15-minute experiment (Figure 3B; Table 1). This reflects the inappropriate formation and retraction of supernumerary pseudopodia in Lsc KO neutrophils.
Stimulation of neutrophils with fMLP triggers an accumulation of phosphatidylinositol-3,4,5-triphosphate (PIP3) and then the serine/threonine kinase Akt at the leading edge of the single-dominant pseudopod.33,34 We used confocal microscopy to demonstrate that PIP3 and Akt also accumulate at the leading edge of pseudopodia in Lsc KO neutrophils (Figure 3C-D). Although PIP3 and Akt are not visible in all pseudopodia of Lsc KO neutrophils, this is also true in WT cells and likely reflects different stages of pseudopod formation. This demonstrates that Lsc is not required for PIP3 or Akt accumulation at the leading edge of fMLP-stimulated neutrophils.
Stimulation of neutrophils with fMLP also triggers an accumulation of phosphorylated myosin light chain (pMLC) and RhoA at the trailing edge of the uropod.3 It has been suggested that impaired formation of the uropod can promote formation of supernumerary pseudopodia.3 We used confocal microscopy to demonstrate that pMLC and RhoA accumulate similarly at the trailing edge of the uropod in fMLP-stimulated Lsc KO and WT neutrophils (Figure 3E-F). This demonstrates that Lsc is not required for accumulation of pMLC or RhoA at the trailing edge of fMLP-stimulated neutrophils.
Lsc is required for normal speed and directionality of formyl-peptide–stimulated neutrophil migration
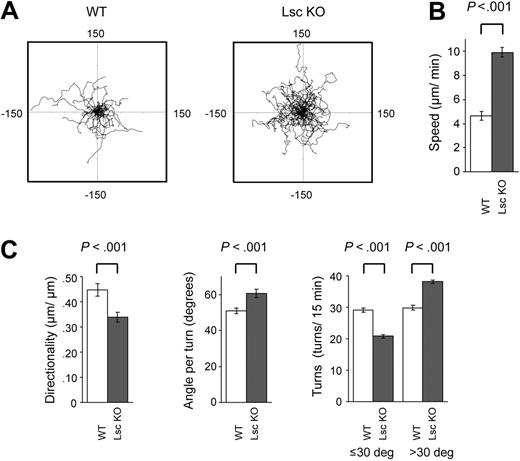
Quantitative analysis of time-lapse photomicrographs collected as described in Figure 3 demonstrated that Lsc KO neutrophils have significantly enhanced chemokinesis; they migrate more than twice as fast as WT neutrophils when stimulated with a uniform concentration of fMLP (Figure 4A-B; Table 1; Supplemental Videos S1-S2). Lsc KO neutrophils also have abnormal directional migration; they have reduced directionality (net distance traveled/linear distance traveled), they make larger directional changes (angle/turn), they make fewer small (< 30°) turns, and they make more large (> 30°) turns compared with WT neutrophils when stimulated with a uniform concentration of fMLP (Figure 4A,C; Table 1; Supplemental Videos S1-S2).
Although Lsc KO neutrophils have abnormal fMLP-stimulated chemokinesis, fMLP-stimulated chemotaxis of WT and Lsc KO neutrophils did not differ in Boyden or Zigmond chamber analysis (data not shown). Together, these results demonstrate that Lsc is required to regulate the speed and directionality of migration in a uniform concentration of fMLP but not for detection or migration along a gradient of fMLP.
Lsc is required for normal β2- and β1-integrin–dependent adhesion of formyl-peptide–stimulated neutrophils
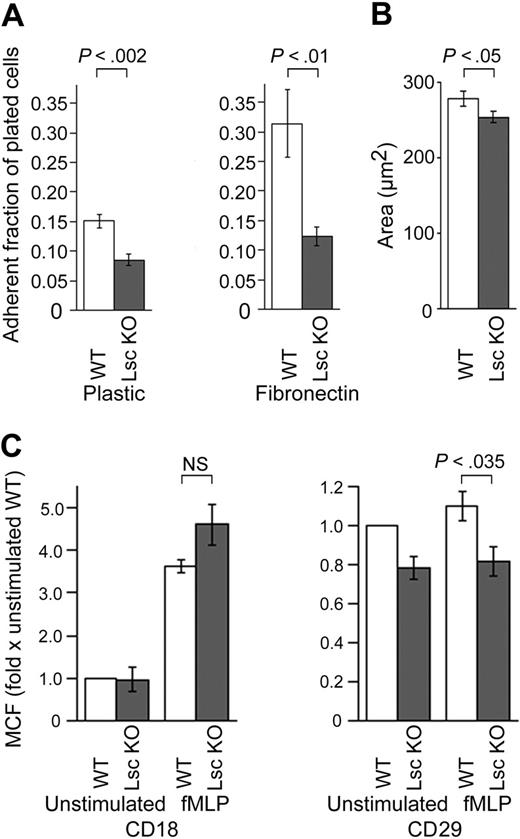
fMLP-stimulated Lsc KO neutrophils are significantly less adherent to both uncoated plastic and fibronectin (Figure 5A). Adhesion to plastic requires normal β2-integrin function,35 and adhesion to fibronectin requires normal β1-integrin function. fMLP-stimulated Lsc KO neutrophils are not elongated, indicating they do not have impaired uropod de-adhesion or retraction (Supplemental Videos S1-S2). Together, these results indicate that Lsc is required for both β2- and β1-integrin–dependent adhesion but not for uropod de-adhesion or retraction. fMLP-stimulated Lsc KO neutrophils are less spread out than WT neutrophils, which is also consistent with reduced adhesion of the Lsc KO neutrophils (Figure 5B).
We compared the surface expression of CD18 (β2-integrin) and of CD29 (β1-integrin) on neutrophils in suspension, before and 30 minutes after stimulation with fMLP. CD18 expression is similar on unstimulated WT and Lsc KO neutrophils (Figure 5C). Stimulation with fMLP increases CD18 surface expression approximately 4-fold on both (Figure 5C). CD29 is expressed at low levels on unstimulated WT and Lsc KO neutrophils, and stimulation with fMLP does not significantly change CD29 expression in either (Figure 5C). Lsc KO neutrophils express approximately 20% less CD29 on their surface, both before and after stimulation with fMLP (Figure 5C). These results demonstrate that β2- and β1-integrin expression on fMLP-stimulated Lsc KO neutrophils are normal and near-normal, respectively. This indicates that integrin affinity and/or avidity are impaired in fMLP-stimulated Lsc KO neutrophils.
Lsc is required to form a single-dominant pseudopod in formyl-peptide–stimulated neutrophils. DIC photomicrographs (25 × objective) of WT and Lsc KO neutrophils stimulated with 10 μM fMLP in a Zigmond chamber were obtained at 15-second intervals for 15 minutes and subjected to quantitative analyses of cell shape (see Supplemental Videos 1-2 and Table 1). (A) Representative sequential photomicrographs of fMLP-stimulated WT and Lsc KO neutrophils. WT neutrophils form and sustain a single-dominant pseudopod (solid arrowhead). In contrast, Lsc KO neutrophils rapidly generate and retract pseudopodia at random locations around the cell perimeter. (B) fMLP-stimulated Lsc KO neutrophils undergo larger cumulative positive and negative changes (Δ) in area, perimeter, and roundness. These differences result from the rapid turnover of supernumerary pseudopodia in Lsc KO neutrophils. Data are the mean ± SEM for 60 cells of each genotype from 3 independent experiments. (C-F) Confocal images (63 ×) of WT and Lsc KO neutrophils adhered to a glass coverslip and incubated with 1 μM fMLP for 5 minutes. The cells were then fixed, permeabilized, and labeled with phalloidin (red) and the indicated antibodies. Bars represent 10 μm. Images are representative of 2 independent experiments. (C-D) fMLP-stimulated PIP3 and Akt accumulation at the leading edge of pseudopodia is similar in WT and Lsc KO cells. PIP3 and Akt are not visible in all pseudopodia of WT or Lsc KO neutrophils, likely reflecting varying stages of pseudopod formation. (E-F) fMLP-stimulated pMLC and RhoA accumulation at the trailing edge of the uropod is similar in WT and Lsc KO cells. Solid arrowhead indicates leading edge; open arrowhead, trailing edge. All photomicrographs are representative of at least 2 independent experiments.
Lsc is required to form a single-dominant pseudopod in formyl-peptide–stimulated neutrophils. DIC photomicrographs (25 × objective) of WT and Lsc KO neutrophils stimulated with 10 μM fMLP in a Zigmond chamber were obtained at 15-second intervals for 15 minutes and subjected to quantitative analyses of cell shape (see Supplemental Videos 1-2 and Table 1). (A) Representative sequential photomicrographs of fMLP-stimulated WT and Lsc KO neutrophils. WT neutrophils form and sustain a single-dominant pseudopod (solid arrowhead). In contrast, Lsc KO neutrophils rapidly generate and retract pseudopodia at random locations around the cell perimeter. (B) fMLP-stimulated Lsc KO neutrophils undergo larger cumulative positive and negative changes (Δ) in area, perimeter, and roundness. These differences result from the rapid turnover of supernumerary pseudopodia in Lsc KO neutrophils. Data are the mean ± SEM for 60 cells of each genotype from 3 independent experiments. (C-F) Confocal images (63 ×) of WT and Lsc KO neutrophils adhered to a glass coverslip and incubated with 1 μM fMLP for 5 minutes. The cells were then fixed, permeabilized, and labeled with phalloidin (red) and the indicated antibodies. Bars represent 10 μm. Images are representative of 2 independent experiments. (C-D) fMLP-stimulated PIP3 and Akt accumulation at the leading edge of pseudopodia is similar in WT and Lsc KO cells. PIP3 and Akt are not visible in all pseudopodia of WT or Lsc KO neutrophils, likely reflecting varying stages of pseudopod formation. (E-F) fMLP-stimulated pMLC and RhoA accumulation at the trailing edge of the uropod is similar in WT and Lsc KO cells. Solid arrowhead indicates leading edge; open arrowhead, trailing edge. All photomicrographs are representative of at least 2 independent experiments.
Dominant role of Lsc in neutrophils is to activate RhoA
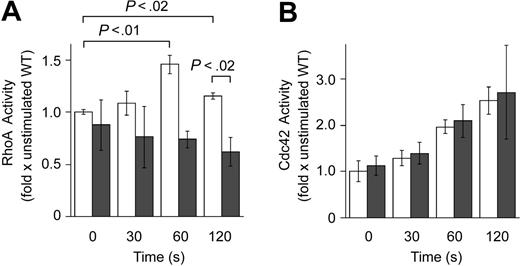
Lsc contains functional domains that can enhance (DH domain) or inhibit (RGS domain) Gα12/13 activation of RhoA. To determine the dominant role of Lsc in neutrophils, we compared RhoA activity in wild-type (WT) and Lsc KO neutrophils. We measured RhoA activity in neutrophils in suspension to eliminate the effects of integrin signaling on RhoA activity.36 There is a significant difference between the RhoA activity in fMLP-stimulated WT and Lsc KO neutrophils. Stimulation with fMLP increases RhoA activity in WT neutrophils by approximately 50% (Figure 6A). In contrast, stimulation with fMLP causes little or no change in RhoA activity in Lsc KO neutrophils (Figure 6A). Peak RhoA activity in fMLP-stimulated WT neutrophils is approximately 1.5-fold that in Lsc KO neutrophils (Figure 6A).
Inhibition of Cdc4237 or Rac134 activity impairs the ability of fMLP-stimulated myeloid cells to form a single-dominant pseudopod. We compared Cdc42, Rac1, and Rac2 activities in fMLP-stimulated WT and Lsc KO neutrophils. The fMLP-stimulated activities of Cdc42, Rac1, and Rac2 were similar in WT and Lsc KO cells (Figure 6B; data not shown). Together, these results demonstrate that Lsc regulates migration and pseudopod formation in fMLP-stimulated neutrophils by altering RhoA, but not Cdc42, Rac1, or Rac2 activities.
Lsc KO mice have a peripheral leukocytosis and extramedullary hematopoiesis
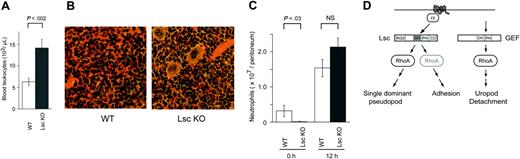
Lsc KO mice have a significant peripheral leukocytosis, with 2-fold the number of circulating neutrophils and lymphocytes as WT mice (Figure 7A; Table 2). In contrast, they have normal peripheral-blood hemoglobin concentrations and platelet counts (Supplemental Table S2). The Lsc KO mice also have significant splenomegaly (Table 3) with extramedullary hematopoiesis in the red pulp of the spleen (Figure 7B). The Lsc KO mice have no evidence of local or systemic infections, are housed in the same facility as WT mice, and have similar life spans as WT mice. Together, these results indicate that Lsc is required for both peripheral neutrophil and lymphocyte homeostasis.
Normal recruitment of Lsc KO neutrophils to E coli peritonitis and chemical dermatitis
At rest, there are significantly fewer neutrophils in the peritoneal cavity of Lsc KO mice compared with WT (Figure 7C). Twelve hours after the inoculation of 106 cfu E coli into the peritoneal cavity, the numbers of neutrophils in the peritoneal cavity of Lsc KO and WT mice are similar (Figure 7C). Lsc KO mice also have normal cellular recruitment, myeloperoxidase activity, and edema at the site of croton oil-induced dermatitis 6 hours after oil application (data not shown). These results indicate that, in contrast to signaling pathways required for leukocyte homeostasis, other signaling pathways can compensate for the Lsc deficiency in some forms of inflammation.
Lsc is required for normal migration of formyl-peptide–stimulated neutrophils. DIC photomicrographs (25 × objective) of WT and Lsc KO neutrophils stimulated with 10 μM fMLP in a Zigmond chamber were obtained at 15-second intervals for 15 minutes as described in Figure 3A and subjected to quantitative analyses of cell migration (see Supplemental Videos S1-S2 and Table 1). (A) Wind-rose plots of fMLP-stimulated WT and Lsc KO neutrophil migration over 15 minutes (n = 60 cells of each genotype). Scale, μm. Lsc KO cells migrate farther and with a more meandering path. (B) fMLP-stimulated Lsc KO neutrophils migrate faster than WT neutrophils. (C) fMLP-stimulated Lsc KO neutrophils migrate with decreased directionality compared with WT neutrophils. The fMLP-stimulated Lsc KO neutrophils also make larger average turns, fewer small turns (≤ 30°), and more large turns (> 30°) turns, compared with WT neutrophils. Data are the mean ± SEM for 60 cells of each genotype from 3 independent experiments.
Lsc is required for normal migration of formyl-peptide–stimulated neutrophils. DIC photomicrographs (25 × objective) of WT and Lsc KO neutrophils stimulated with 10 μM fMLP in a Zigmond chamber were obtained at 15-second intervals for 15 minutes as described in Figure 3A and subjected to quantitative analyses of cell migration (see Supplemental Videos S1-S2 and Table 1). (A) Wind-rose plots of fMLP-stimulated WT and Lsc KO neutrophil migration over 15 minutes (n = 60 cells of each genotype). Scale, μm. Lsc KO cells migrate farther and with a more meandering path. (B) fMLP-stimulated Lsc KO neutrophils migrate faster than WT neutrophils. (C) fMLP-stimulated Lsc KO neutrophils migrate with decreased directionality compared with WT neutrophils. The fMLP-stimulated Lsc KO neutrophils also make larger average turns, fewer small turns (≤ 30°), and more large turns (> 30°) turns, compared with WT neutrophils. Data are the mean ± SEM for 60 cells of each genotype from 3 independent experiments.
fMLP-stimulated Lsc KO neutrophils are less adherent. (A) Neutrophils plated on either plastic or fibronectin were stimulated with 10 μM fMLP for 30 minutes and washed 3 times, and the remaining adherent neutrophils were quantified as a fraction of the total plated. Data are the mean ± SEM calculated from 6 independent samples in each experiment. Plots are representative of at least 2 independent experiments. (B) The adherent area of WT and Lsc KO neutrophils plated on a glass coverslip and stimulated with 10 μM fMLP in a Zigmond chamber are as described in Figure 3A. Lsc KO neutrophils are less spread out than WT neutrophils. (C) Surface expression of CD18 (β2-integrin) and CD29 (β1-integrin) on fMLP-stimulated WT and Lsc KO neutrophils. Neutrophils in suspension were incubated with 10 μM fMLP or vehicle alone for 30 minutes and labeled with an anti-CD18 or anti-CD29 antibody, and the geometric mean cellular fluorescence (MCF) of the labeled neutrophils was measured by flow cytometry and normalized to the MCF of unstimulated WT neutrophils. Data are the mean normalized MCF ± SEM from 3 independent experiments. CD18 expression is similar in WT and Lsc KO neutrophils before and after stimulation. CD29 expression is reduced in Lsc KO neutrophils before and after fMLP-stimulation.
fMLP-stimulated Lsc KO neutrophils are less adherent. (A) Neutrophils plated on either plastic or fibronectin were stimulated with 10 μM fMLP for 30 minutes and washed 3 times, and the remaining adherent neutrophils were quantified as a fraction of the total plated. Data are the mean ± SEM calculated from 6 independent samples in each experiment. Plots are representative of at least 2 independent experiments. (B) The adherent area of WT and Lsc KO neutrophils plated on a glass coverslip and stimulated with 10 μM fMLP in a Zigmond chamber are as described in Figure 3A. Lsc KO neutrophils are less spread out than WT neutrophils. (C) Surface expression of CD18 (β2-integrin) and CD29 (β1-integrin) on fMLP-stimulated WT and Lsc KO neutrophils. Neutrophils in suspension were incubated with 10 μM fMLP or vehicle alone for 30 minutes and labeled with an anti-CD18 or anti-CD29 antibody, and the geometric mean cellular fluorescence (MCF) of the labeled neutrophils was measured by flow cytometry and normalized to the MCF of unstimulated WT neutrophils. Data are the mean normalized MCF ± SEM from 3 independent experiments. CD18 expression is similar in WT and Lsc KO neutrophils before and after stimulation. CD29 expression is reduced in Lsc KO neutrophils before and after fMLP-stimulation.
Lsc is required for normal RhoA activity in formyl-peptide–stimulated neutrophils. (A) RhoA activity (activated RhoA/total RhoA) was measured in suspended WT and Lsc KO neutrophils incubated with 10 μM fMLP for the indicated times. Data are the mean RhoA activity ± SEM normalized to the RhoA activity of unstimulated WT neutrophils from 3 independent experiments. Formyl-peptide stimulation increases RhoA activity in WT neutrophils. Formyl-peptide–stimulated RhoA activity is decreased in Lsc KO neutrophils compared with WT neutrophils. (B) Cdc 42 activity (activated Cdc42/total Cdc42) is similar in suspended WT and Lsc KO neutrophils incubated with 10 μM fMLP for the indicated times. Data are the mean Cdc42 activity ± SEM normalized to the Cdc42 activity of unstimulated WT neutrophils from 2 independent experiments. □ indicates WT; ▪, KO.
Lsc is required for normal RhoA activity in formyl-peptide–stimulated neutrophils. (A) RhoA activity (activated RhoA/total RhoA) was measured in suspended WT and Lsc KO neutrophils incubated with 10 μM fMLP for the indicated times. Data are the mean RhoA activity ± SEM normalized to the RhoA activity of unstimulated WT neutrophils from 3 independent experiments. Formyl-peptide stimulation increases RhoA activity in WT neutrophils. Formyl-peptide–stimulated RhoA activity is decreased in Lsc KO neutrophils compared with WT neutrophils. (B) Cdc 42 activity (activated Cdc42/total Cdc42) is similar in suspended WT and Lsc KO neutrophils incubated with 10 μM fMLP for the indicated times. Data are the mean Cdc42 activity ± SEM normalized to the Cdc42 activity of unstimulated WT neutrophils from 2 independent experiments. □ indicates WT; ▪, KO.
Lsc KO mice have peripheral-blood leukocytosis and extramedullary hematopoiesis, with normal recruitment of neutrophils to E coli peritonitis. (A) The mean number of peripheral-blood leukocytes ± SEM in adult WT and Lsc KO mice (n = 11 age- and sex-matched mice of each genotype) (see Table 2). The mean peripheral leukocyte count in Lsc KO mice is 2-fold that of WT mice. (B) Extramedullary hematopoiesis in the red pulp of the spleen. Light micrographs (25 × objective) of spleen sections from WT and Lsc KO mice stained with hematoxylin and eosin. Representative of 4 mice of each genotype. (C) Lsc KO mice have significantly fewer peritoneal neutrophils at rest, but similar numbers of peritoneal neutrophils 12 hours after intraperitoneal injection of 106 cfu E coli. Data are the mean number of peritoneal neutrophils ± SEM for at least 4 mice at each time point. (D) Model for the role of Lsc in formyl-peptide–stimulated neutrophils. Lsc is required to form a single-dominant pseudopod and for normal adhesion in formyl-peptide–stimulated neutrophils. Schematic diagram of Lsc signaling pathways in neutrophils (see “Discussion” for details). Formyl-peptides bind 7-transmembrane segment surface receptors. The ligand-bound receptors release activated G-protein α-subunits that stimulate the Lsc DH domain to activate RhoA. Activated RhoA is required to form a single-dominant pseudopod. Lsc is also required for formyl-peptide–stimulated neutrophil adhesion. Lsc may regulate adhesion through a RhoA-independent pathway. Lsc is not required for RhoA-dependent uropod release.
Lsc KO mice have peripheral-blood leukocytosis and extramedullary hematopoiesis, with normal recruitment of neutrophils to E coli peritonitis. (A) The mean number of peripheral-blood leukocytes ± SEM in adult WT and Lsc KO mice (n = 11 age- and sex-matched mice of each genotype) (see Table 2). The mean peripheral leukocyte count in Lsc KO mice is 2-fold that of WT mice. (B) Extramedullary hematopoiesis in the red pulp of the spleen. Light micrographs (25 × objective) of spleen sections from WT and Lsc KO mice stained with hematoxylin and eosin. Representative of 4 mice of each genotype. (C) Lsc KO mice have significantly fewer peritoneal neutrophils at rest, but similar numbers of peritoneal neutrophils 12 hours after intraperitoneal injection of 106 cfu E coli. Data are the mean number of peritoneal neutrophils ± SEM for at least 4 mice at each time point. (D) Model for the role of Lsc in formyl-peptide–stimulated neutrophils. Lsc is required to form a single-dominant pseudopod and for normal adhesion in formyl-peptide–stimulated neutrophils. Schematic diagram of Lsc signaling pathways in neutrophils (see “Discussion” for details). Formyl-peptides bind 7-transmembrane segment surface receptors. The ligand-bound receptors release activated G-protein α-subunits that stimulate the Lsc DH domain to activate RhoA. Activated RhoA is required to form a single-dominant pseudopod. Lsc is also required for formyl-peptide–stimulated neutrophil adhesion. Lsc may regulate adhesion through a RhoA-independent pathway. Lsc is not required for RhoA-dependent uropod release.
Discussion
Lsc KO neutrophils have markedly abnormal polarization in response to fMLP stimulation (Figure 3; Table 1; Supplemental Videos S1-S2). They exhibit 2 abnormal behaviors. First, they form pseudopodia at inappropriate locations around the entire perimeter of the cell, rather than at a single location. Second, they are unable to sustain a pseudopod, once it is formed. Inhibition of Gα12/13 or RhoAcauses fMLP-stimulated HL-60 cells to form supernumerary pseudopodia around the cell perimeter, similar to fMLP-stimulated Lsc KO neutrophils.3 Lsc belongs to a small group of Rho GEFs that link surface receptor–associated G-protein α-subunits to RhoA.13 Here, we demonstrate that RhoA activity in fMLP-stimulated Lsc KO neutrophils is significantly less than in WT neutrophils. Peak fMLP-stimulated RhoA activity in WT neutrophils is approximately 1.5-fold the peak activity in Lsc KO neutrophils (Figure 6A). Together, the similarity of the polarization defect in Lsc KO neutrophils and Gα12/13 or RhoA-inhibited HL-60 cells (Figure 3), the ability of Lsc to bind and act as an effector for Gα12/13,19 the RhoA-substrate specificity of Lsc,17 and the decreased RhoAactivity in Lsc KO neutrophils (Figure 6A) suggest that Lsc couples formyl-peptide receptors to RhoA signaling pathways required to form a single-dominant pseudopod in neutrophils (Figure 7D).
In vitro, Gα12/13 can stimulate the Lsc DH domain to activate RhoA,19 whereas the Lsc RGS domain can inhibit Gα12/13.18,25 Here, we demonstrate that the net effect of Lsc in formyl-peptide–stimulated neutrophils is to activate RhoA (Figure 6A). This indicates that the DH domain is the dominant functional domain in coupling formyl-peptide receptors to RhoA in neutrophils. Our results do not exclude the possibility that the RGS domain modulates the magnitude or timing of RhoA activation. It is also possible that another Gα-subunit present in neutrophils, such as Gα16, couples formyl peptide receptors to Lsc.
Published reports of the effect of fMLP on RhoA activation in myeloid cells differ. Gakidis et al38 state that fMLP stimulation did not increase RhoA activity in mouse neutrophils. Chang et al39 present data that demonstrate RhoA activity is approximately 3-fold baseline in fMLP-stimulated rat neutrophils. Alblas et al4 present data that demonstrate RhoA activity is at least 2-fold baseline in fMLP-stimulated human neutrophils. Xu et al3 present data that demonstrate RhoA activity is approximately 2.5-fold baseline in fMLP-stimulated HL-60 cells. We found that stimulation of mouse bone marrow neutrophils increases RhoA activity by 50% (Figure 6A). This is a substantial increase but does not rule out the possibility that Lsc may also have RhoA-independent effects on neutrophil polarization. Gα12/13 can signal in a RhoA-independent manner,40,41 but this has not been described for Lsc.
Currently, the downstream details of how Lsc and RhoApromote the formation of a single-dominant pseudopod are not fully understood. RhoA and the activated form of its downstream effector, MLC, migrate to the uropod of fMLP-stimulated HL-60 cells.3 Because inhibition of RhoA or myosin II in fMLP-stimulated HL-60 cells promotes supernumerary pseudopodia formation, this suggested the hypothesis that RhoA actively promotes uropod formation, and as a result, indirectly inhibits inappropriate pseudopod formation.3 We have demonstrated that formyl-peptide–stimulated Lsc KO neutrophils have a normal distribution of RhoA and phosphorylated pMLC in the uropod. This makes it unlikely that abnormal RhoA or pMLC accumulation in the uropod is responsible for the abnormal pseudopod formation in Lsc KO neutrophils (Figure 3E-F). An alternate hypothesis is that Lsc is required at the leading edge for normal pseudopod formation and that abnormal pseudopodia are produced in its absence. We have demonstrated that formyl-peptide–stimulated Lsc KO neutrophils accumulate PIP3, Akt, and F-actin normally in their pseudopodia (Figure 3C-D). This indicates that Lsc is not required for initial PIP3 or subsequent Akt accumulation. It does not rule out the possibility that Lsc affects pseudopod formation and/or stabilization downstream of Akt recruitment. Recent work indicates that fMLP-stimulated Rac1-deficient neutrophils also have supernumerary pseudopodia34 and that Cdc42 is required to sustain pseudopodia in fMLP-stimulated HL-60 cells.37 These results raise the possibility that Lsc participates in a common signaling pathway with Rac1 and/or Cdc42 to regulate pseudopod formation and maintenance. We have demonstrated that formyl-peptide–stimulated WT and Lsc KO neutrophils have similar amounts of activated Cdc42 and Rac1 (Figure 6B; data not shown). This indicates that Lsc is not required for formyl-peptide–stimulated Cdc42 or Rac1 activation, but it does not rule out the possibility that Lsc functions in parallel with or downstream of Cdc42 and/or Rac1 to regulate pseudopod formation.
RhoA is required for neutrophil de-adhesion and uropod detachment.4-6 Because Lsc is a RhoA GEF, we predicted Lsc KO neutrophils would have increased adhesion. Unexpectedly, fMLP-stimulated Lsc KO neutrophils have reduced β2- and β1-integrin–dependent adhesion (Figure 5A). Because these results suggest that Lsc and RhoA have opposite effects on neutrophil adhesion, Lsc may also regulate adhesion in a RhoA-independent manner (Figure 7D). An alternative explanation is that the primary role of Lsc in the signaling pathways that regulate neutrophil adhesion is to inhibit RhoA, likely by inhibiting Gα12/13 through the Lsc RGS domain. Because total RhoA activity is reduced in Lsc KO cells (Figure 6A), this seems less likely.
We found no evidence that uropod detachment or retraction is inhibited in Lsc KO neutrophils (Supplemental Videos S1-S2). Activated RhoA is reduced but not absent in Lsc KO neutrophils (Figure 6A), demonstrating that formyl-peptides can activate RhoA in the absence of Lsc. Together, these results indicate that uropod detachment and retraction are controlled by an Lsc-independent RhoA signaling pathway that could use one of the other G-protein α-subunit–associated Rho GEFs (eg, LARG) (Figure 7D). It is also possible that impaired adhesion of the entire Lsc KO neutrophil masks defects in uropod de-adhesion and retraction.
Lsc KO neutrophils have abnormal chemokinesis in response to fMLP; they migrate faster in a uniform concentration of the chemoattractant (Figure 4A-C). Cell migration speed can be enhanced by impaired cell adhesion.42 Lsc KO neutrophils have impaired adhesion (Figure 5A), and this may be responsible for their increased migration speed. In contrast to chemokinesis, chemotaxis of WT and Lsc KO neutrophils to fMLP is similar. Together, these results demonstrate that Lsc is required to regulate the speed and directionality of migration in a uniform concentration of chemoattractant but not for detection or migration along a gradient of chemoattractant. This indicates that Lsc-independent signaling pathways activated by a formyl-peptide gradient can compensate for Lsc-dependent signaling pathways activated by a uniform concentration of formyl-peptide. fMLP-stimulated neutrophils lacking the Rho GEFs Vav1 and Vav3 also have impaired adhesion to β2-integrin ligands, are less spread out, and migrate faster than WT neutrophils when stimulated with fMLP.38 Despite these defects, like Lsc KO neutrophils, Vav1- and Vav3-deficient neutrophils also have normal chemotaxis to fMLP in vitro.38 This raises the possibility that Lsc and Vav1 and Vav3 participate in a common signaling pathway that regulates adhesion but is not absolutely required for chemotaxis.
Lsc KO mice have a peripheral leukocytosis, splenomegaly, and extramedullary hematopoiesis (Figure 7A; Tables 2, 3). This disordered leukocyte homeostasis in Lsc KO mice is particularly interesting because of the strong association between impaired leukocyte adhesion molecule function and peripheral leukocytosis in humans and mice. Humans with decreased CD18 expression or function,43 impaired synthesis of fucosylated glycan selectin ligands,44,45 or impaired chemokine-dependent integrin activation46,47 all have impaired neutrophil adhesion and a peripheral leukocytosis. Mice lacking adhesion molecules, including CD18,48 CD11a,49 P- and E-selectin,50 ICAM-1,51 or one of several enzymes required for selectin ligand synthesis,52-54 also have a peripheral leukocytosis with variable elevations in the neutrophil and lymphocyte counts. The etiologies of these leukocytoses in humans and mice are unknown, but in some cases they have been correlated to elevated levels of hematopoietic growth factors. CD18-deficient mice have increased serum concentrations of IL-3,48 IL-6,48 IL-17,55 and G-CSF.55 CD18-deficient neutrophils also have a defect in apoptosis that may play a role in their altered homeostasis.56 Other mechanisms that have been postulated for these leukocytoses in humans and mice with impaired leukocyte adhesion are a decrease in the fraction of the circulating leukocyte pool that is adhered to the vessel wall and impaired transmigration of leukocytes from the peripheral blood. This strong association between impaired adhesion and leukocytosis raises the possibility that impaired leukocyte adhesion may be the cause of the leukocytosis in Lsc KO mice. Currently, we are trying to understand how trafficking of hematopoietic precursor cells is affected in Lsc KO mice.
Recruitment of Lsc KO neutrophils in models of E coli peritonitis and chemical dermatitis is normal. This indicates that other signaling pathways may compensate for the absence of Lsc in some inflammatory systems. Elegant experiments with CD18-/- mice demonstrate that different mechanisms of neutrophil recruitment are required for different types of inflammation.57 CD18-/- mice have impaired recruitment to chemical dermatitis but normal recruitment to bacterial peritonitis.57 It may be that other signaling pathways are not able to compensate for Lsc in other forms of inflammation.
Prepublished online as Blood First Edition Paper, November 1, 2005; DOI 10.1182/blood-2005-03-1164.
Supported by a Clinical Investigator Development Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health (K08-04080) (D.J.L.), a Grant-in-Aid from the American Heart Association (0455858T) (D.J.L.), The New York Chapter of the Arthritis Foundation (D.J.L.), a Glorney-Raisbeck Fellowship from the New York Academy of Medicine (S.A.F. and P.K.), and the John C. Sable Memorial Heart Fund (S.A.F.).
S.A.F. and X.S. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Shaun R. Coughlin for his support of this project; Yao Wu Zheng and Wei Huang for technical assistance; Robert V. Farese Jr for generously providing the RF8 sv129Jae ES cells; and Geoffrey W. Abbott, Lynda Pierini, Shahin Rafii, and Carl Nathan for helpful discussions of this manuscript.
Supplemental data
Lsc KO bone marrow neutrophils stimulated with fMLP. Images were acquired and assembled into a video as described in the legend for Video 1. This video is representative of 6 similar videos acquired in 3 independent experiments. (See Materials and Methods for details).