Percutaneous minimally invasive radiofrequency (RF) ablation has not been described for lymphoma. This image-guided modality is presented in 3 different settings for the treatment of refractory lymphoma. The first patient received RF ablation for the curative treatment of a solitary residual hepatic mass following rituximab-based chemotherapy for a posttransplantation lymphoproliferative disorder (PTLD) and is disease-free 4 years later. The second patient received RF ablation for successful palliation of progressive follicular lymphoma adjacent to the bladder wall following chemotherapy and maximum radiation. The third patient received RF ablation for prevention of airway obstruction from progressive diffuse large B-cell lymphoma of the right neck following chemotherapy and maximum radiation. RF ablation may be clinically beneficial and should be considered for the treatment of local lymphoma that is refractory or not amenable to standard approaches.
Introduction
External radiation therapy (XRT) is the preferred method for local control of lymphoma masses. However, its application is limited by radiation tolerance of surrounding normal tissues and it may be associated with significant morbidity. Occasionally, lymphomas may also display radiation resistance requiring alternative treatment strategies. Clinical results suggest radiofrequency (RF) ablation may be an excellent alternative to surgery or radiation in selected settings, but its role for lymphoma is undocumented. RF ablation is a percutaneous method of tissue destruction that has been used as an alternative to surgery for the treatment of focal solid tumor lesions up to several centimeters in size. For RF ablation, needlelike electrodes are placed directly into the tumor to enable delivery of RF alternating current from a generator in a minimally invasive fashion without damaging adjacent vital structures. With the patients under conscious sedation or general anesthesia, needles are typically placed with computed tomography (CT) and/or ultrasound guidance and tissue is heated to destructive temperatures.1 RF ablation is well documented for primary and secondary hepatic tumors. It is also under investigation for breast, lung, and kidney tumors as well as pain palliation of soft-tissue tumors and metastatic bone disease.2,3 Herein we describe 3 patients with local lymphoma, refractory to or unable to receive conventional XRT, who were successfully treated with RF ablation.
Study design
All patients were enrolled on National Institutes of Health (NIH) Institutional Review Board–approved protocols and gave informed consent. The Radionics Cosman CC-1 Coagulator 200-watt, 480-kHz generator ablation system (Valleylab, Boulder, CO) was used with a triple-needle probe (Valleylab) under local anesthesia with conscious sedation for 2 patients and under general anesthesia for one patient.
Case no. 1: refractory posttransplantation lymphoproliferative disorder
A 30-year-old man who had received a cadaveric kidney transplantation presented with stage IV Epstein-Barr virus–associated polymorphic posttransplantation lymphoproliferative disease (PTLD). After tacrolimus and mycophenolate were withdrawn and dose-adjusted–etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH)–rituximab (DA-EPOCH-R) chemotherapy was initiated, he achieved complete resolution of lung disease but had a persistent metabolically active lesion measuring 3.3 × 3.3 cm in the liver.4,5 Due to the hepatic location, RF ablation was chosen as the preferred treatment and performed for a total of 36 minutes in 3 different locations without complication. The patient remains without evidence of disease 4 years after RF ablation.
Case no. 2: refractory indolent lymphoma
A 54-year-old male presented with left leg swelling, deep venous thrombosis, and right iliac lymphadenopathy secondary to a large left pelvic mass. Biopsy showed follicular center cell lymphoma. He was treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy followed by radiation to a residual pelvic mass. Three years later he relapsed in the pelvis and received cyclophosphamide and fludarabine. A second relapse 3 years later was treated with DA-EPOCH-R. Later he began to experience suprapubic pain, and a magnetic resonance imaging (MRI) revealed a 7.0 × 5.7 × 5.2-cm mass adjacent to the left bladder wall, which was within a prior maximum radiation field.
RF ablation was performed for a total of 21 minutes at 2 locations without complication. An MRI obtained the following day showed a large peribladder mass with a 4.8 × 3.8 × 4.5-cm central thermal lesion. A positron emission tomography (PET) scan 5 weeks later showed persistent metabolic activity surrounding the thermal lesion but the patient denied clinical symptoms. At 3 months follow-up, the patient continued to deny clinical symptoms and had returned to normal daily activities.
Six months after the first ablation, CT and MRI revealed enlargement of the left pelvic bladder mass to 9.7 × 11.3 × 7.7 cm. A second ablation was performed at 7 locations with a total treatment time of 20 minutes without complication. Four cycles of rituximab were given after this ablation treatment. A follow-up CT scan revealed a larger area of central necrosis measuring 7.1 × 7.6 × 5.4 cm in the bladder mass. Although his symptoms resolved for 6 months after RF ablation, he subsequently developed a contralateral recurrence requiring further chemotherapy and radiation.
Case no. 3: refractory transformed lymphoma
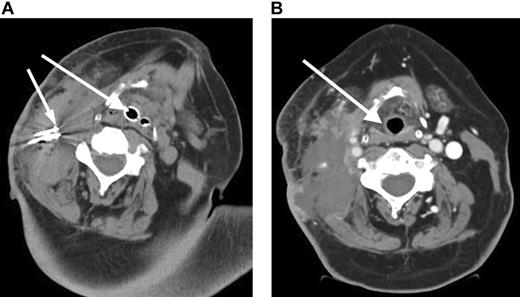
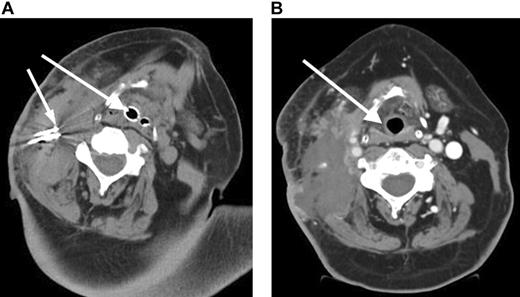
A 57-year-old male presented with stage I follicular center cell lymphoma. He received CHOP with rituximab followed by 3000 cGy radiation with complete resolution of the neck mass. A relapse occurred 2 months later, and a fine-needle aspiration revealed a histologic transformation of his disease in the neck. He received an additional cycle of CHOP followed by ICE (ifosfamide, carboplatin, etoposide) chemotherapy and an additional 3600 cGy to the neck.6 He then received a reduced-intensity allogeneic stem cell transplant.7 However, he later developed progressive neck pain and a rapidly enlarging neck mass (Figure 1A). Due to impending airway obstruction, maximum prior radiation, and the patient's refusal to undergo a tracheostomy, RF ablation combined with liposomal doxorubicin to increase tissue coagulation and intratumoral drug accumulation was performed at 4 different locations in the right neck without complication.8 Because of the location of the mass and the potential for worsening airway obstruction, the patient was observed in the intensive care unit following the RF ablation. Subsequently, the patient achieved resolution of his symptoms (Figure 1B) but died 5 weeks later from his disease progression at other sites.
Results and discussion
Radiation is the preferred treatment for local control of neoplasms that are chemotherapy refractory or require local palliation. However, it is limited by tissue tolerance and may produce end organ toxicity.9 Additionally, neoplasms may display radiation resistance and require alternative treatment approaches. Approximately one third of patients undergoing RF ablation may experience a transient postablation syndrome consisting of flulike symptoms that can be treated conservatively. These symptoms are generally related to the volume of tissue ablated. Other side effects may include a transient elevation of liver transaminases, pain, and nausea.10 RF ablation represents an important therapeutic modality that can be performed in several hours, may reduce cost and inconvenience to the patient, and is not limited by tissue radiation tolerance. However, RF ablation does have treatment size limitations, and its efficacy in lymphoma is unknown. While RF ablation is being used for tumors in the kidney, lung, and bone, and for pain palliation, it is primarily used for unresectable liver tumors due to multifocal disease. Currently, tumors smaller than 5 cm are considered the best indication for RF ablation, although larger lesions can be treated by overlapping ablations.11
We describe 3 lymphoma patients who successfully received RF ablation for different clinical scenarios. In the first patient, RF ablation eradicated a chemotherapy-resistant focus of PTLD, and the patient remains free of disease. This case represents a unique setting in which control of isolated chemotherapy-resistant disease, presumably representing expansion of a resistant EBV+ clone, can lead to long-term disease control and likely cure. In the second patient, RF ablation was used to control symptomatic local disease that was refractory to radiation and chemotherapy. This case also illustrates that RF ablation can be successfully repeated to control local disease. In the third patient, RF ablation prevented airway obstruction and tracheostomy in a patient with radiation refractory disease and significantly improved the patient's quality of life.
A hypothetical and unexplored use of RF ablation is to promote a tumor-specific immune response. RF ablation induces a robust inflammatory response in the tumor bed that, in the presence of idiotype vaccine and/or immunostimulants, may be an adjuvant for boosting an antitumor immune response. In situ tumor models of tumor destruction in mouse B16-OVA melanoma show that tumor debris is a potential antigen source for the induction of antitumor immunity.12 In this setting, RF ablation of B16-OVA results in a clear delay in the outgrowth of B16-OVA tumor cells and a low level of protection in 20% of the mice. Further study reveals that antitumor reactivity is found to be transferred to naive mice by splenocytes and directed against multiple tumor antigens.12 Another example of immune system modulation is seen with RF ablation of VX2 liver tumors in a rabbit model that produces shrinkage of remote metastases, raising the possibility of an immunostimulatory mechanism.13 A similar rabbit model demonstrated a tumor-specific T-lymphocytic reaction following RF ablation.14 Such an immune response indicates that RF ablation combined with immunomodulation can induce a systemic immune response and therefore might enhance the effectiveness of RF ablation treatment and protect against local recurrences and metastasis. Future studies will need to define the potential combination of RF ablation and immunotherapy.
Rapidly enlarging large B-cell lymphoma neck mass causing airway obstruction. (A) Pre-RF ablation CT scan shows a large right cervical mass with extensive tracheal deviation with compressed airway expanded by endotracheal tube (large arrow) and RF ablation needles (small arrow) within cervical mass. (B) Post-RF ablation CT demonstrates a devascularization and reduction in cervical mass and improvement of tracheal deviation with shift of trachea (arrow) to midline.
Rapidly enlarging large B-cell lymphoma neck mass causing airway obstruction. (A) Pre-RF ablation CT scan shows a large right cervical mass with extensive tracheal deviation with compressed airway expanded by endotracheal tube (large arrow) and RF ablation needles (small arrow) within cervical mass. (B) Post-RF ablation CT demonstrates a devascularization and reduction in cervical mass and improvement of tracheal deviation with shift of trachea (arrow) to midline.
These cases illustrate 3 different settings in which RF ablation may be an acceptable treatment option for lymphoma, producing potentially less morbidity than standard treatments. RF ablation may be helpful in lymphoma for pain palliation, chemoresistant and radiation-resistant disease, debulking, and protecting adjacent structures (like airway) from local effects of tumor growth. However, our experience is limited and the results are relatively short term. Further studies are needed to define the clinical indications for RF ablation in lymphoma.
Prepublished online as Blood First Edition Paper, October 27, 2005; DOI 10.1182/blood-2005-05-2131.
Supported in part by the Intramural Research Program of the National Institutes of Health, Clinical Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.