Abstract
Epstein-Barr virus (EBV) is a tumorigenic herpes virus that infects and persists in B lymphocytes in the majority of humans, generally without causing disease. However, in a few individuals the virus is associated with significant pathology, particularly benign and malignant lymphoproliferations. Recently acquired knowledge on the mechanisms of EBV persistence, immune control of primary and persistent infection, and disease pathogenesis is now being translated into the clinic with novel methods of diagnosis, prevention and treatment contributing to improved patient care. This review concentrates on these recent advances in the field of hematology/oncology.
Introduction
Epstein Barr virus (EBV) was first identified in 1964, in cultures from a biopsy of a Burkitt lymphoma.1 Shortly afterward its potent transforming ability was demonstrated in cell culture when the virus was shown to induce blast transformation and uncontrolled proliferation of infected B lymphocytes.2,3 This in vitro-transforming ability reflects the oncogenic potential of the virus, which has since been associated with a number of malignancies (Table 1).
EBV is a very successful member of the herpes virus family, infecting greater than 90% of the world's adult population. Like all herpes viruses, EBV is able to persist in the host for life, but in the vast majority of healthy carriers the virus causes no disease.4 This is because a delicate balance is maintained between the host immune system, which limits production of virus particles, and the virus, which persists and is successfully transmitted in the face of host antiviral immunity. Disruption of this balance, resulting from to primary or acquired immunodeficiency, may lead to the development of EBV-associated disease (Table 1).
During the past 5 years there has been a substantial increase in our understanding of both the biology and immune control of EBV in the healthy host, the molecular mechanisms involved in viral proliferation, and oncogenesis. This progress has provided the building blocks for development of novel therapeutic approaches that are now making a significant impact on clinical management of EBV-associated lymphoid malignancies. In this review we discuss the clinical disease associations of EBV, focusing on areas in which recent advances in our understanding of disease pathogenesis are leading to change in patient management. These include life-threatening primary infection and the EBV-associated malignancies posttransplantation lymphoproliferative disease and Hodgkin disease.
Infectious mononucleosis
Infectious mononucleosis (IM) is generally the result of delayed primary infection with EBV and is characterized by the triad of fever, lymphadenopathy, and pharyngitis. It typically occurs in young adults but is also occasionally seen in children and older adults. IM is rare in nonindustrialized countries, where EBV infection almost invariably occurs in early childhood. A classic feature of IM is an absolute blood lymphocytosis that consists mainly of CD8+ T cells, and it is this immune response which is thought to cause the symptoms of IM.
The epidemiology of IM was established in the 1950s,5 and EBV was identified as the causative agent in 1968.6 Following this, several seroepidemiologic studies were carried out that identified IM as a disease of the high social classes in Westernized societies and found an incidence among young adults undergoing primary EBV infection ranging from 26% to 74%.7-10 In a study on more than 2000 university students, 75% were EBV positive at entry to university, with a significantly greater number of EBV-positive women (79.2%), compared with men (67.4%; P < .001). Seventeen percent of students reported a history of IM, with previous sexual activity being a strong risk factor.11 These findings suggest that EBV transmission occurs during sexual intercourse, but, although there are reports of EBV in genital secretions of both male and female asymptomatic carriers,12-14 more detailed studies are required to assess the significance of these findings to viral transmission and IM. Of the 25% EBV-negative students at university entry, approximately 50% seroconverted while at university, and 23% of these developed IM. It remains unclear why only a proportion of late seroconverters develop clinical disease, but it may be related to genetic factors that control the host response and/or the dose of virus received at initial infection.
Classic acute IM resolves in 2 to 6 weeks, but relapses can occur in the first 6 to 12 months following infection, and IM may be linked, in the short term, with a prolonged fatigue syndrome and depression.15 The risk of these complications seems to be higher in those with premorbid psychological and social problems and with lower physical fitness.16 However, there is no evidence that the chronic fatigue syndrome is caused by abnormal immunologic response to EBV.17
Whether EBV initially infects B lymphocytes or squamous epithelial cells when transmitted orally is still debated, but early in IM foci of EBV-infected B lymphoblasts can be seen in the interfollicular regions of the tonsil.18 Detailed examination of viral gene expression in these infected tonsillar B cells has led to the definition of a model for primary infection and the establishment of EBV persistence.19 In this model EBV drives infected B cells down the B-cell activation/maturation pathway, mirroring physiologic antigen activation by changing patterns (programs) of latent viral gene expression at key points along the way. Thus, EBV-infected naive tonsillar B cells undergo proliferation (driven by the viral growth program), traverse the germinal center (protected from apoptosis by the default program), and mature into memory cells (expressing the latency program) (Table 2).
After infected memory B cells are released into the circulation, viral proteins are only rarely expressed.20 During IM up to 50% of the memory B-cell population may be EBV infected,21 and this reservoir of latently infected cells allows for long-term EBV persistence.
The key feature of the immune response in IM is the marked expansion of activated (atypical) lymphocytes, and much progress has been made in characterizing the scale, phenotype, and epitope specificity of these cells.22,23 Typically, most activated lymphocytes in IM (greater than 70%) are CD8+ cytotoxic T lymphocytes (CTLs), which express the activation markers such as HLA-DR, CD45RO, CD38.24-26 This dramatic CD8+ T-cell response is thought to both control the infection by lysis of proliferating EBV-infected B cells and to cause the symptoms of IM by excessive cytokine release.27,28 Thus, levels of TH1 type cytokines such as interleukin (IL) 2 and interferon (INF) γ are elevated, and recent studies show a correlation between the level of activated T cells and the severity of the symptoms in IM.29
The scale of the CD8+ lymphocytosis in IM has promoted debate as to whether these cells are superantigen driven or represent a large clonal/oligoclonal response to specific viral antigens.30 Analysis of diversity in T-cell receptors is widely accepted as identifying the majority of these cells as antigen specific,25 and by using tetramer technology the same group showed that up to 40% of the peripheral blood CD8+ cytotoxic T cells may be directed against a single viral epitope.31
Data are more limited on the response of other lymphocyte subsets during IM. However, recent work shows that CD4+ T cells, although not significantly increased in number in IM, have an activated phenotype and specifically respond to lytic viral epitopes.29,32 In addition, NK cells may play an important role in the control of primary EBV infection by eliminating infected B cells and augmenting the antigen specific T-cell response via release of immunomodulatory cytokines.33
X-linked lymphoproliferative disease
Males with the rare X-linked lymphoproliferative (XLP) disease are unable to control primary EBV infection; greater than 50% die during the acute phase of the disease, and most of the survivors later develop hypogammaglobulinemia and/or malignant lymphoma. In 1998, 3 groups, taking quite different approaches, identified the genetic defect underlying XLP,36-38 and this has allowed accurate diagnostic testing for the syndrome, which may vary markedly in its clinical presentation. In addition, substantial progress has been made in understanding the pathogenesis of XLP, which in turn has led to new insights into the control of primary EBV infection in healthy individuals.
The onset of clinical disease in boys with XLP is generally abrupt. In response to primary EBV infection they rapidly develop a severe IM-like syndrome characterized by massive polyclonal expansion of EBV-infected B cells, which in turn stimulates an uncontrolled proliferation of cytotoxic T cells. Unlike the primary response to EBV in healthy individuals, the T cells are apparently unable to limit the proliferation of B cells, and this dysregulated cytotoxic T-cell response and subsequent cytokine release leads to extensive organ damage.39 Bone marrow failure secondary to hemophagocytosis remains the most common cause of death in XLP. The other major clinical phenotypes and associated mortality are shown in Table 3.40
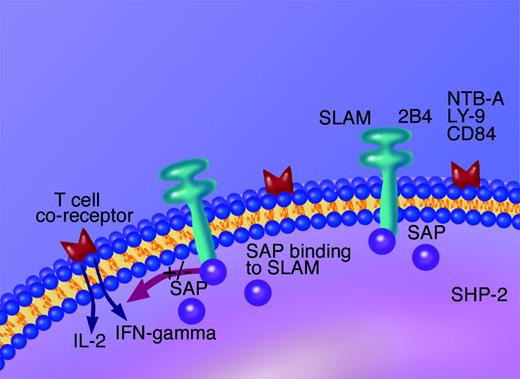
The mutated/deleted protein in XLP, signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) (also called SH2D1A), was first identified as a cytoplasmic signal transduction protein for cell-surface SLAM/CD150.37 SAP is a short adaptor molecule of 128 aa (amino acids) that contains one SH2 domain and is expressed in activated T and NK cells. SAP binds to SLAM, a member of the immunoglobulin (Ig) superfamily which is primarily expressed on T and B lymphocytes and dendritic cells; expression is rapidly up-regulated on both CD4+ and CD8+ T-cell subsets following activation in vitro.41 Engagement of SLAM by specific antibody leads to T-cell proliferation and preferential production of TH1 type cytokines, including INFγ.42
SAP functions as a cytoplasmic regulator of cellular activation through SLAM and at least a further 4 members of the Ig superfamily including 2B4/CD244,43 CD84 and Ly-9,44 and NTB-A.45 By binding to phosphorylated SLAM, SAP may prevent signaling by other SH2 domain-containing proteins (such as SHP-2), which have downstream signaling capacity (Figure 1).
However, more recent work using mouse models shows that SAP is a modulatory molecule that facilitates recruitment of FynT tyrosine kinase to the phosphorylated cytoplasmic domain of SLAM.46,47 The recruitment of FynT allows tyrosine phosphorylation signaling that is essential for TH2 cytokine production by activated T cells.48 Thus, it is thought that variations in the level of SAP-SLAM binding alter the balance of TH1/TH2 cytokine production, with an absence of SAP, as in XLP, allowing excessive production of TH1 cytokines such as INFγ.
Diagram showing the mechanism of action of SAP. SAP is an intracellular modulator of lymphocyte activation, both via the T-cell co-receptor SLAM or NK cell co-receptor 2B4/CD244. SAP is proposed to check binding of other SHP2 containing domains and thus inhibit downstream cell activation and cytokine release.
Diagram showing the mechanism of action of SAP. SAP is an intracellular modulator of lymphocyte activation, both via the T-cell co-receptor SLAM or NK cell co-receptor 2B4/CD244. SAP is proposed to check binding of other SHP2 containing domains and thus inhibit downstream cell activation and cytokine release.
2B4/CD244 is a costimulatory receptor that is universally expressed on all NK cells from healthy individuals,49,50 and it is a key intracellular transducer of NK cell-activation signals, again controlled by SAP. Interestingly, NK cell lines with mutations in SAP, cloned from individuals with XLP, fail to kill EBV-infected B-lymphoblastoid cell lines (LCLs).51 In vitro, CD244 binds CD48,52 a molecule that is highly expressed by B lymphocytes shortly after infection with EBV,53 and it has been suggested that this CD244/CD48 interaction may provide a mechanism for NK cell recognition of EBV-infected cells which is absent in XLP. Thus, excessive production of TH1 cytokines from activated T cells may cause the symptoms of XLP, and failure of NK cell control of primary EBV infection, because of abnormal signaling via 2B4/CD244, may explain the close link with EBV.
The collection of data by the XLP registry has allowed important analysis of the spectrum of clinical features that occur in XLP, and, since identification of the gene, new diagnostic criteria for the diagnosis of XLP have been established (Table 4).54
Diagnostic and prenatal screening of putative cases is normally carried out by polymerase chain reaction (PCR) amplification and direct sequencing of all 4 exons of the SAP gene. This has revealed some individuals with a typical clinical presentation and good family history in whom no mutation is found, suggesting a mutation outside the coding region. For this reason screening for protein expression may be the preferred option.55 As well as the more typical presentations, screening of males with common variable immunodeficiency has identified previously unsuspected cases of XLP. The mechanism of hypogammaglobinemia in these XLP cases has been clarified using mice with mutations in the Sap gene. B cells from these mice fail to switch Ig isotype and mature into memory B cells following virus infection as a result of the lack of appropriate help from SAP-deficient CD4+ T cells.56 Interestingly, this defect in B-cell maturation may also provide continuing antigenic stimulation, driving the uncontrolled T-cell activation during primary EBV infection in XLP, since down-regulation of latent viral gene expression is linked to the B-cell maturation pathway.19
As a number of cases of genetically confirmed XLP have been identified with clinical features, usually lymphoma development, prior to primary EBV infection, it is clear that precipitating factors of the XLP syndrome other than EBV (perhaps other virus infections) must exist.
Interestingly, no relation has been found between XLP genotype and phenotype, and in particular those with large genomic deletions do not appear to have worse disease than those with single nucleotide substitutions.57 It has also been noted that among families with the same mutation very variable clinical presentations may occur, suggesting that additional environmental or virologic factors must contribute to the clinical outcome.
The treatment of XLP remains difficult, and the only curative option is allogeneic hemopoietic stem cell transplantation, as reviewed by Gaspar et al.39 Identification of the gene has allowed accurate screening of family members, and regular Ig infusions may protect those identified as at risk of developing clinical XLP. Additionally, Milone et al58 hypothesized that as the clinical features of XLP are caused by a dysregulated T-cell response secondary to an absence of functional SAP, rapid elimination of EBV-infected B cells may ameliorate the clinical syndrome. They therefore treated 2 males with known germ line mutations in SAP, at the time of primary EBV infection, with the anti-CD20 monoclonal antibody, rituximab. This led to rapid resolution of clinical features, loss of detectable EBV DNA within circulating lymphocytes, and no progression of disease within 2 years of treatment.58
Chronic active EBV
Chronic active EBV (CAEBV) is characterized by chronic or recurrent IM-like symptoms (fever, lymphadenopathy, fatigue, hepatosplenomegaly), which are life threatening. The cause is unknown, but mutations in the perforin gene have recently been identified in one patient with CAEBV.59 Typically, the clinical course is less acute than fulminant IM, but the long-term prognosis is poor. The disease is most commonly reported from Japan, and a national survey carried out by Kimura et al60,61 has helped to clarify the key features. The diagnostic criteria for CAEBV used in the survey included a longer then 3-month duration of EBV-related illness and a high viral load in the peripheral blood or a grossly abnormal EBV antibody profile. In total 82 patients were identified who fulfilled these criteria and had been diagnosed since 1990.
Like XLP, CAEBV predominately occurs in children, and Kimura et al60 found the mean age of onset was 11 years (range, 9 months to 53 years). The mortality was high, at greater than 40%, with a mean survival of only 4.3 years from onset. The causes of death included hepatic failure, lymphoma, hemophagocytic syndrome, and complications of transplantation.60 Kimura et al61 also made the important observation that, unlike healthy virus carriers, T and NK cells are infected with EBV, and the type of cell infected could be linked to prognosis. Those with T-cell CAEBV had a shorter survival time than those with NK cell disease.61 Another prognostic feature was the viral load, with higher viral loads found in those with severe disease. These data may be useful in guiding treatment options, but no definitive treatment regimens have been established. Antiviral drugs and immunosuppressive agents (etoposide, cyclosporine, steroids) have all been used but without clear evidence of success. Stem cell transplant may be successful,62,63 but should be reserved for those with a poor prognosis because the risk of transplantation is substantial. Another new treatment option is infusion of autologous EBV-specific CTLs; in a small study, it induced remission in the majority of patients treated.64
Posttransplantation lymphoproliferative disease
Classically, PTLD is an EBV-driven opportunistic B lymphoproliferation occurring in individuals in which iatrogenically suppressed T-cell immunity is unable to control primary or persistent EBV infection. Consequently, the level of immunosuppression is a major risk factor for PTLD, and because this varies with the type of organ transplanted, the incidence of PTLD varies widely (< 1%-33%). The highest incidence occurs following heart/lung, liver/small bowel, and other multiorgan transplantations (< 33%); the lowest is reported following stem cell transplantations (< 1%), with kidney, liver, and heart transplantations having an intermediate incidence (1%-3%).34
Around 50% of PTLDs are associated with primary EBV infection, and these tend to occur early after transplantation, present with IM-like symptoms, and are particularly common in children (who are more likely than adults to be EBV negative at the time of transplantation).65,66 However, the term PTLD covers a spectrum of B-lymphoproliferative lesions, and EBV-negative lesions are now well recognized, particularly among those occurring late after transplantation when presentation is typically of a single nodal or extranodal lesion.67,68 The World Health Organization classification recognizes 3 histologic categories of PTLD: hyperplastic, polymorphic, and lymphomatous or monomorphic. It has been suggested that these categories represent stages in progression of PTLD, because monomorphic tumors are the most aggressive and resistant to treatment, and have often accumulated genetic abnormalities such as mutations of c-myc, N-ras, BCL-6, and/or P53.69
EBV is clearly a key factor in the pathogenesis of EBV-positive PTLD, and may be the sole requirement for outgrowth of the hyperplastic, polyclonal lesions associated with primary EBV infection in which the proliferating B lymphoblasts express all the viral genes required to drive B-cell transformation (Table 2). However, this is not always the case; viral gene expression may be heterogeneous both within and between tumors, with a minority of PTLDs showing the more-restricted pattern of latent viral gene expression typical of Hodgkin disease or Burkitt lymphoma (Table 2).70 In healthy individuals approximately 1 to 50 per million circulating B cells are EBV infected, and this is increased in most immunocompromised transplant recipients. Yet PTLD only develops in a minority and often presents as a single, clonal lesion. This suggests that only a few EBV-infected cells have the capacity to proliferate and that other factors in addition to EBV must be required for PTLD development. Recent findings have gone some way to identifying these factors.
Whereas persistent EBV infection in healthy carriers is restricted to the memory cell compartment in peripheral blood,19 analysis of somatic mutations in Ig heavy chain genes in PTLD lesions has shown that both naive and memory B cells may give rise to PTLD. Furthermore, when tumors arose from memory B cells, frequent Ig gene mutations were found, suggesting that EBV had infected an abnormal cell, provided survival signals in the germinal center, and induced its proliferation.71 Other suggested cofactors in PTLD development include chronic antigenic stimulation by the allograft which, through cytokine production by activated CD4 T cells (interleukin [IL] 4, 6, 10), may promote B-cell growth. This suggestion is supported by the frequent finding of infiltrating CD4 T cells in PTLD biopsy material,72 and by experiments in severe combined immunodeficient mice, showing that CD4 T cells are required for the outgrowth of EBV-positive PTLD-like tumors from peripheral blood B cells.73
PTLD responds better to treatment in its early stages, when it can be difficult to diagnose because its presentation may resemble infection or graft rejection. Therefore, good patient monitoring, prevention procedures, and improved treatment regimens are essential. Significant advances have been made in this area during recent years from the accumulated knowledge about the EBV-specific CTL response and the pathogenesis of PTLD.
Although no control trials have been carried out, and no standard protocols are yet available, several studies suggest that monitoring the viral load in whole blood, mononuclear cells, or plasma has predictive value for PTLD.74-76 Most transplantation centers now monitor EBV load using real-time PCR and regard a high value as indicative of a high risk of PTLD. Data from several groups show that viral load after stem cell transplantation (SCT), particularly if the graft was T-cell depleted, has some positive, and a very good negative, predictive value.74,75 It is now standard practice to treat a high EBV load following SCT with the monoclonal anti-CD20 antibody rituximab, which rapidly lowers the viral load by eliminating circulating B cells. This treatment has been shown to reduce the incidence of PTLD in small studies when compared with historic controls.77
The predictive value of EBV load in solid organ transplantation (SOT) is less clear because many recipients have high EBV loads and remain stable for months or years without developing PTLD.78,79 Therefore, regular monitoring is advised to detect a rapid rise, which is believed to be more significant than a single high value. Monitoring is particularly important in those who are EBV seronegative at the time of transplantation and may therefore experience primary EBV infection early after transplantation, especially if the organ donor was EBV positive. At present the data for SOT do not support the use of preemptive therapy, and large detailed studies using standardized techniques to detect EBV DNA are necessary to define the precise risks and optimize the monitoring procedures.
There are several treatment options for PTLD (Table 5), but no randomized controlled trails have been undertaken to determine the best regimen, and mortality remains high at around 50%. Recent advances in our understanding of the immune mechanisms controlling primary and persistent EBV infection have led to new and exciting immunotherapeutic approaches to PTLD therapy.
PTLD is an ideal tumor to trial T-cell immunotherapy, because it is an EBV-driven lymphoproliferation in which the tumor cells express the known targets for CD8+ T cells that control the persistent infection in healthy individuals (Table 2). Additionally, early treatment of PTLD following SOT with reduction of immunosuppression achieves tumor regression in a significant proportion of cases, presumable by allowing reactivation of the CD8+ T-cell response. Adoptive T-cell immunotherapy for PTLD in SCT was pioneered by Rooney et al,80 who showed that infusions of EBV-specific CTLs grown in vitro from bone marrow donors could successfully prevent and treat PTLD in recipients of stem cell transplants. Infused cells survived up to 18 months in recipients and were shown to proliferate in response to reactivation of EBV infection.81 However, the situation is more complex in SOT than in SCT (Table 6), and studies are more limited. SOT donors are generally neither available nor suitable to provide CTLs, but some benefit has been reported from the use of autologous CTLs, albeit without significant in vivo survival or expansion.82-84
To circumvent the time-consuming and expensive nature of CTL therapy tailored to individual patients, and to provide treatment for EBV-negative recipients of solid organ transplants who are at high risk of PTLD, we have developed a bank of 100 CTLs grown from healthy blood donors,85 which is being used on a best HLA match basis in an ongoing trial to treat PTLD. In a pilot study of patients with progressive PTLD unresponsive to conventional treatments, 3 of 5 who completed the course of infusions showed complete remission. No graft-versus-host disease was detected, and graft function improved in those who responsed.86 Furthermore, in a recently reported case of EBV-positive primary cerebral lymphoma in a child with primary immunodeficiency, a dramatic and full recovery followed infusions of allogeneic CTLs.87 This latter case indicates that infused CTLs can access the central nervous system and, because the child had no functional EBV-specific T cells, strongly supports the direct role of the infused CTLs in tumor response. Thus, although long-term survival of the allogeneic CTLs is not expected,86 this therapy appears safe and beneficial and is amenable to widespread use against EBV- and other virus-associated tumors.
Hodgkin disease
An association between EBV and Hodgkin disease (HD) has long been suspected; IM was linked with a 3-fold increase of risk of developing HD in 1980,88 and later high antibody titers to EBV lytic cycle antigens were shown to predate HD diagnosis.89 Additionally, EBV clonal DNA has been identified in tumor cells90 and localized to the malignant Reed Sternberg cells (RSCs).91 Approximately 40% to 50% of HDs are EBV positive, with the strongest association with the mixed cellularity subtype. HD in the immunocompromised and in the developing world shows the strongest links to EBV; in contrast, in the West the EBV association is strongest with HD in young children and the elderly. Surprisingly, in HD in young adults, the risk group for IM, only around 30% of tumors are typically reported as EBV positive. However, in a very extensive study the risk of HD and EBV status of the tumor were monitored in 38 555 patients with laboratory-confirmed IM.92 The virus was found in 55% of the tumors, a much higher rate than previously reported, and the risk of EBV-positive HD was increased 4-fold following IM, with a median incubation period of 4.1 years. At present there is conflicting data on whether EBV status alters prognosis; in some subgroups, such as males and young adults, EBV-positive status may be linked to improved disease-free survival, but larger prospective studies are required.93,94
The role of EBV in HD pathogenesis is not entirely clear, because RSCs express only a subset of the latent viral genes required for B-cell transformation in vitro (Table 2).95 However, in vivo these genes (EBNA1, LMP1, LMP2) are thought to play a vital role in EBV persistence by protecting infected B cells from apoptosis in the germinal center.19 It is now generally accepted that RSCs represent postgerminal center B cells, but that IgG molecules are not expressed, often because of functional defects in their Ig gene regulatory elements.96 These findings suggest that EBV infection provides survival signals for abnormal B cells in the germinal center and drives their proliferation. The similarities between these findings in HD and PTLD suggest a common initiating step in tumor pathogenesis.
One of the puzzles about EBV-positive HD has been that RSCs express high levels of the immunogenic proteins LMP1 and LMP2 and yet are not targeted by CTLs. In this regard generalized immunosuppression is a well-recognized feature of HD, and, although it is poorly characterized, recent evidence suggests that regulatory T cells present in blood and tumor tissue may render the T cells anergic.97
Current treatment protocols for HD are very effective, with complete remission rates of up to 90%. But approximately one third of patients relapse, and other treatment options are then required. Following on from the use of adoptive CTL therapy in PTLD, interest has developed in using this treatment strategy in EBV-positive HD. Although RSCs do not express the immunodominant EBNA3 proteins, CTL specific for EBNA-1, LMP-1, and LMP-2 can be generated in vitro, which may overcome the anergic state. A detailed phase 1 study in which 14 patients with relapsed HD were treated with autologous EBV-specific CTLs recorded complete remission in 5 patients up to 40 months after treatment.98 The infused cell lines were shown to recognize LMP-2, expand in vivo, and traffic to tumor sites. Additionally, a pilot study, taking a similar approach, treated 6 patients with allogeneic EBV CTLs and also documented measurable disease responses.99
Vaccine development
Both primary and persistent EBV infections are associated with significant pathology which an effective vaccine could potentially prevent. EBV infects B cells via binding of the major viral envelope glycoprotein 350 (gp350) to the CD21 receptor on resting B cells,100,101 and during primary infection neutralizing antibody to gp350 is generated. gp350 vaccines are under trial primarily aimed at preventing IM in the first instance. The first trial to be reported was a small study in China involving 19 children, 9 of whom received a live recombinant vaccinia virus expressing gp350.102 Six of the 9 children remained EBV negative 16 months after vaccination, compared with the 10 control subjects who all seroconverted during this time period. Although these results are promising, use of live vaccinia virus vaccines is generally considered unacceptable by the licensing authorities.
Now a phase 2, randomized, double-blind, placebo-controlled trial using gp350 in an alum-based adjuvant is under way. One hundred eighty EBV-negative Belgian university students have been enrolled in the study, which aims to evaluate the efficacy of the vaccine by determining the attack rate of IM in vaccine and control groups during an 18-month period.103 Results are expected by the end of 2005, but at this time there are many unanswered questions regarding the strategy for an EBV vaccine (Table 7).
It is important that an EBV vaccine is entirely safe, because in the majority of healthy people primary and persistent EBV infections cause no disease. In this regard there are concerns over the duration of the immune response induced, because, if used in children, a vaccine could merely delay primary infection and thereby convert a potential subclinical childhood infection into IM.
CTL-based vaccines are also being developed as therapeutic agents for EBV-linked tumors,104 and work has focused on the development of LMP-specific vaccines, because LMP-1 and LMP-2 are the key latent antigens expressed in EBV-associated nasopharyngeal carcinoma and HD.104-106
Prepublished online as Blood First Edition Paper, October 18, 2005; DOI 10.1182/blood-2005-07-2702.