Comment on Mitsiades et al, page 1092
Rapid development of preclinical findings brings renewed hope to clinical practice in the fight against multiple myeloma.
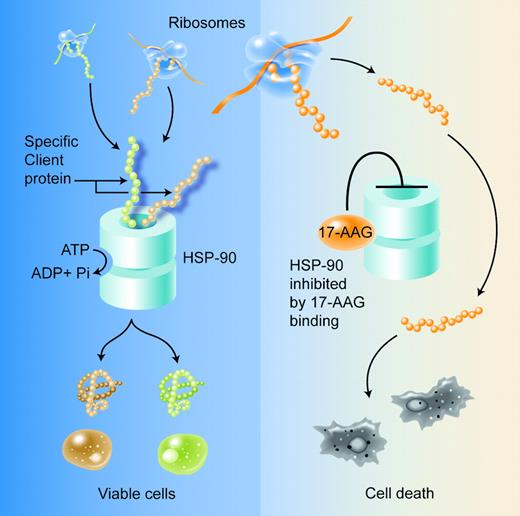
In this issue, Mitsiades and colleagues report on yet another compound with potential therapeutic effectiveness in multiple myeloma. HSP-90 is a molecular chaperone required for the conformational maturation of several known oncogenic signaling proteins that support malignant cell growth and survival. Inhibition of the HSP-90 complex compromises the posttranslational maturation of these proteins, thereby reversing the survival advantage conferred by their oncogenic activity (see figure).1
The specific set of HSP-90 client proteins known to date (HER2/neu, p53, ER, bcr/abl, Raf-1, Akt) has focused the use of HSP-90 inhibitors in tumor types where these are presumed to be biologically relevant.2 While Raf-1 and Akt activation have been associated with IL-6 signaling and proliferation of myeloma cells,3 data presented in this article suggest that the antiproliferative and proapoptotic effect of the novel HSP-90 inhibitor 17-allyl-aminogeldanamycin (17-AAG) in myeloma cells is mediated through undefined HSP-90 client proteins. The authors report the effects of HSP-90 inhibition on several candidate signaling pathways important in multiple myeloma, including insulin-like growth factor receptor-1 (IGF-1R), interleukin-6 receptor (IL-6R), IKK/NF-κB, PI-3K/Akt, and Raf/MAPK, as well as downstream effects on the proteasome and telomerase. Identification of these new HSP-90 clients suggests a broader use for HSP-90 inhibitors in additional tumor types as well as novel rational therapeutic combinations for targeting multiple pathways concurrently.
Binding of 17-AAG at the ATP binding site of HSP90 results in functional inhibition of the chaperone. This results in misfolding and destabilization of the client proteins essential for survival of the malignant cell. Misfolded proteins are degraded through the ubiquitin-proteasome pathway. Illustration by A. Y. Chen.
Binding of 17-AAG at the ATP binding site of HSP90 results in functional inhibition of the chaperone. This results in misfolding and destabilization of the client proteins essential for survival of the malignant cell. Misfolded proteins are degraded through the ubiquitin-proteasome pathway. Illustration by A. Y. Chen.
These findings are critical as they open another potential therapeutic avenue for myeloma treatment. An intriguing approach reported in this article is the ability to design treatment combinations based on molecular signals. In fact, ongoing clinical trials are exploring the role of 17-AAG either alone or in combination with bortezomib in patients with relapsed or refractory myeloma. Preliminary results of these studies are promising and have already reported antimyeloma activity and clinical benefit to patients. Of interest, responses to combination therapy are reported in patients who have failed either agent alone.4,5
While multiple myeloma remains incurable, identifying the range of underlying molecular defects that contribute to myeloma cell survival and resistance to treatment, as well as unveiling changes in the microenvironment that facilitate the progression of myeloma, is key to the future of drug discovery in myeloma. Such efforts have already resulted in the approval of one compound (bortezomib), with the second (lenalidomide) knocking at the Food and Drug Administration's doors for approval. With the passionate support of patient advocate groups such as the Multiple Myeloma Research Foundation (MMRF) and the International Myeloma Foundation (IMF), a concerted effort is under way to fight myeloma. Encouraging findings with molecularly targeted therapeutics and rational combinations, such as those reported by Mitsiades et al, along with rapid translation to clinical practice, may soon render traditional high-dose chemotherapy and autologous stem cell transplantation a thing of the past. These reports herald a new dawn of hope for patients with multiple myeloma. ▪