Abstract
Heterogeneity of the effector functions displayed by rituximab and other anti-CD20 monoclonal antibodies (mAbs) apparently recognizing the same CD20 epitope suggests that additional mechanisms, probably related to mAb fine specificity, are responsible for B-cell depletion. To improve our understanding of rituximab's function, its fine specificity was investigated by means of phage display peptide library (PDPL)-expressing 7-mer cyclic (c7c) or 7-/12-mer linear peptides. Rituximab-specific c7c PDPL-derived clone insert sequences expressed the motif A(S)NPS overlapping the human CD20 170ANPS173. P172 was the most critical for rituximab binding, since its replacement with S172 (of mouse CD20) abolished the reactivity. The WPXWLE motif expressed by the linear PDPL-derived clone insert sequences could only be aligned to the reverse-oriented 161WPXWLE156 of acid sphingomyelinase-like phosphodiesterase 3b precursor (ASMLPD), though linear peptides bearing WPXWLE competed with cyclic ones for rituximab-paratope binding. Anti-CD20 mAb 1F5 only displayed a reactivity profile similar to that of rituximab, which also reacted with ASMLPD-derived peptides. Peptides induced antibodies with specificity and effector functions similar to those of rituximab. Our results show a unique fine specificity of rituximab, define the molecular basis for the lack of rituximab reactivity with mouse CD20 (mCD20), and the potential of targeting CD20 in an active immunotherapy setting. A possible rituximab interaction with ASMLPD is suggested.
Introduction
CD20 antigen is a 33- to 35-kDa phosphoprotein expressed at high density on B lymphocytes from the early pre-B to the late B-cell stage, whereas its expression is lost following differentiation of B cells into plasma cells.1 Features that make CD20 attractive as a target for passive immunotherapy are its expression in more than 80% of B-cell lymphomas2 and the lack of its down-regulation upon monoclonal antibody (mAb) binding.3
CD20 sequence analysis predicts a 4-transmembrane domain with intracellular termini and only 1 extracellular 44-amino acid loop (from amino acid 142 to 182), which is the contact site of most anti-CD20 mAbs available,4 including rituximab, which has proven effective for the treatment of lymphoma5,6 or even certain autoimmune diseases.7-9 Antibody-dependent cell-mediated cytotoxicity (ADCC),10 complement-dependent cytotoxicity (CDC),11 and induction of apoptosis12,13 have all been postulated as explanations of B-cell ablation in vivo14 and the therapeutic effect of rituximab. Even so, there is convincing evidence that these mechanisms do not completely explain the cytotoxic effect of rituximab and hence that other unidentified mechanisms, which probably depend on its fine specificity, may operate following mAb infusion.15 Support for this view is provided by the heterogeneity of the effector functions16,17 displayed by rituximab and other anti-CD20 mAbs (ie, mAb B1), which apparently recognize the same CD20 epitope.4
To provide a fuller picture of rituximab's function, we have employed the phage display peptide library (PDPL) technology to illustrate its fine specificity and compared it with that of a panel of previously characterized anti-CD20 mAbs with other effector functions.
The rituximab-specific cyclic and linear synthetic peptides described here have been used to define this mAb's CD20-specific contact site and reveal a unique reactivity profile and its possible cross-reactivity with an amino acid stretch of acid sphingomyelinase-like phosphodiesterase 3b precursor (ASMLPD; genetic identifier number [gi] 7656908).
Materials and methods
Animals
Female BALB/c mice (8 to 12 weeks old) were purchased from Charles River Breeding Laboratory (Milan, Italy).
Cells
The human B-lymphoid CD20+ cell lines Raji and Daudi and the T-lymphoid cell line CEM (CD20-) were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (Hyclone, South Logan, UT; complete medium) and 5 mM L-glutamine.
Conventional reagents, mAbs, and PDPL
Electrophoresis reagents were purchased from Bio-Rad Laboratories (Hercules, CA). Unless otherwise specified, all chemicals were purchased from BDH Chemicals (Poole, United Kingdom). The anti-CD20 rituximab (chimeric IgG1) and its isotype-matched TNFα-specific mAb infliximab were purchased from IDEC Pharmaceutical Corporation (San Diego, CA) and Centocor (Malvern, PA), respectively. The anti-CD20 mAbs B1 (Coulter, Hialeah, FL), 1F5 (ECACC, Salisbury, United Kingdom), LT20,17 CAT13.6,4 and AT8016 were a kind gift from Dr M.S. Cragg (Southampton, United Kingdom). The anti-CD20 mAb 2H7 was purchased from Alexis (Lausen, Switzerland). The anti-CD4 mAb HP2/618 and the HP2/6-specific phagotope HP41-C are available in our laboratory. The anti-HLA class I mAb HC-10-specific peptide Qp1a was characterized previously.19 Purified rabbit IgG, horseradish-peroxidase (HRP)-conjugated avidin (HRP-avidin), and HRP- or FITC-xeno-antibodies to human or mouse IgG (Fc portion) were purchased from Jackson Immunoresearch Laboratories (Avondale, PA). HRP-anti-M13 mAb was purchased from Pharmacia-LKB Biotech AB (Uppsala, Sweden).
The mAbs were purified from ascites by sequential precipitation with caprylic acid and ammonium sulfate20 or by affinity chromatography on protein G (or protein A)-Sepharose (Pharmacia-LKB). F(ab′)2 fragments were obtained by pepsin digestion of chimeric rituximab and infliximab as previously described.18 Their purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).21 The mAb concentration was determined in a bicinchoninic acid assay (Pierce, Rockford, IL). Purified mAb was coupled to biotin using the biotin-N-hydroxysuccinimide ester (Sigma-Aldrich, St Louis, MO) as previously described.22
The c7c, 7-, and 12-mer PDPLs were purchased from New England Bio Lab (Beverly, MA). These are filamentous phage display systems containing a repertoire of 1.2 × 109 sequences of random 7- or 12-amino acid peptides fused to the N-terminal sequence of the M13 synthetic minor-coat protein PIII (5 for each virion), via the flexible linker GGGS, with an overall sequence PFYSHS-X-GGGSA (7- and 12-mer PDPLs) or PFYSHSAC-X-CGGS (c7c PDPL). The 7- and 12-mer PDPLs consist of randomized linear peptides, the c7c PDPL consists of randomized 7-mer peptides, each flanked by a pair of cysteine residues that spontaneously form a disulfide bond. This is useful for targets whose naive ligand is in the context of a surface loop.
Affinity selection, immunoscreening, Western blot, and sequence analysis
Biopanning of PDPLs with rituximab was performed as described,19 the only difference being that infliximab was used as the rituximab isotype-matched mAb to remove allotype- and isotype-specific phage particles.
Enzyme-linked immunosorbent assay (ELISA) screening of phage clones was performed exactly as previously reported,19 whereas Western blot was performed as previously described23 with minor modifications. In brief, purified phage particles (1 × 1010 plaque-forming units [pfu's]/lane) were electrophoresed onto a 10% SDS polyacrylamide gel under nonreducing conditions with the miniProtean II apparatus (Bio-Rad) at a constant 200V for 30 minutes. Resolved proteins were transferred to a polyvinylidene difluoride (PVDF) filter (Millipore, Billerica, MA) with a semidry electrophoresis transfer cell (Bio-Rad) by applying a current of 0.2 A for 30 minutes. After blockade of its free protein active-binding site with PBS containing 0.5% Tween-20, the filter was incubated for 2 hours at 25°C with an appropriate dilution of biotinylated mAb (dissolved in PBS containing 0.2% Tween 20), rinsed in PBS for 5 minutes, incubated with HRP-conjugated avidin, and developed with a diaminobenzidine substrate. Nucleotide sequence analysis of rituximab-specific phage clones was performed as previously described.19
Synthesis of peptides
Cyclic and linear peptides were synthesized at the Sigma (Sigma-Genosys, Cambridge, United Kingdom) and Primm (San Raffaele, Milan, Italy) “peptide synthesis service.” Their quality was determined by analytical reverse-phase chromatography and mass spectral analysis. Their purity was greater than 80%.
KLH conjugation of peptides
Peptides were coupled to KLH by mixing 2 mg peptide with an equal amount of KLH in PBS (final volume 1 mL) in the presence of glutaraldehyde (0.025% final concentration). After a 1-hour incubation at 25°C, the reaction was stopped by extensively dialyzing the mixture against PBS at 4°C. KLH-conjugated peptides (KLH peptides) were stored at -20°C until used.
Immunochemical assay
Unless otherwise indicated, all incubation steps were performed at 25°C. PBS, PBS containing 0.05% Tween 20 (PBS-T20), and PBS containing 0.5% BSA (PBS-BSA) were used as the coating, washing/dilution, and blocking buffers, respectively.
The indirect binding assay to test the reactivity of mAbs with peptides and the cross-blocking assay to determine the ability of synthetic peptides to inhibit the binding of rituximab to them were performed in 96-well polyvinyl-chloride microtiter plates (Falcon; Becton Dickinson, Mountain View, CA) as described,19 with minor modifications.
In the indirect binding assay, plates were coated with peptide by overnight incubation at 4°C of 50 μL/well PBS solution containing 5 μg/mL KLH peptide. Following 2 washings and blockade of free protein-binding sites, 50 μL PBS-T20 solution containing different concentrations of mAbs was added to each well. After a 4-hour incubation and 3 washings, binding of mAbs to peptide was detected by sequential addition of an appropriate dilution of affinity-purified HRP-xeno-antibodies to mouse or human IgG (Fc portion; 90-minute incubation at 25°C) and a freshly prepared o-phenylenediamine-H2O2 (OPD)-substrate solution. The color reaction was stopped with 100 μL/well 2 N H2SO4. Absorbance was read at 492 nm in a Multiscan plate reader (Benchmark; Bio-Rad).
The cross-blocking assay was performed by mixing 50 μL PBS solution containing a 2-fold dilution of peptide (starting concentration 400 μg/mL) with an equal volume of PBS-T20 containing the highest dilution of rituximab giving 90% of the maximal binding (3 μg/mL). Following a 1-hour incubation, the mixture was added to plates previously coated with KLH peptide. After a 4-hour incubation, wells were washed and rituximab binding to peptide was detected with an appropriate dilution of affinity-purified HRP-xeno-antibodies to human IgG (Fc portion). The assay was then continued as described for the indirect binding assay. Results are expressed as percentage of inhibition calculated using the following formula: % inhibition = ([A492 in the absence of inhibitor - A492 in the presence of inhibitor]/A492 in the absence of inhibitor) × 100.
Cell assays
ELISA. The inhibition assay to determine the ability of synthetic peptides to inhibit the binding of mAbs to CD20+ Raji cells was performed in 96-well polyvinyl-chloride microtiter plates (Falcon) by mixing 50 μL of PBS-BSA solution containing 2-fold serial dilutions of inhibitor with an equal volume of an appropriate dilution of biotinylated rituximab. Following a 2-hour incubation at 4°C, the mixture was added to target cells (7 × 105/well) and incubation was prolonged for 2 hours at 4°C. Next, cells were washed twice with PBS, and the binding of biotinylated mAbs to the corresponding antigen was detected by sequential addition of HRP-avidin and OPD-substrate solution. A solution of 2 N H2SO4 (100 μL/well) was used to stop the reaction. Absorbance was read at 492 nm. Results are expressed as percentage of inhibition of binding compared with binding in the absence of inhibitor.
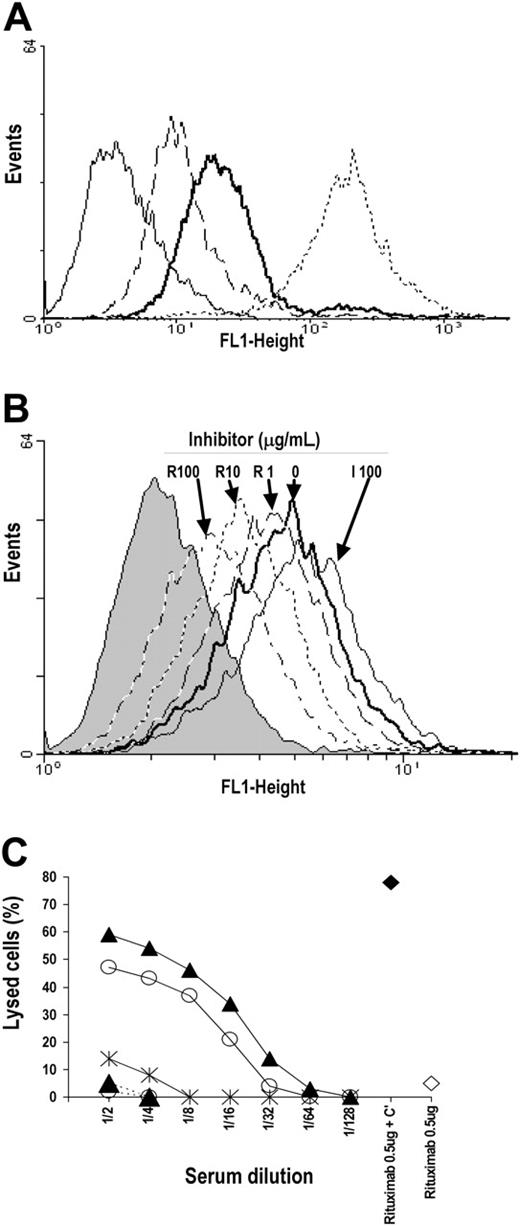
Immunofluorescence. The reactivity of antipeptide serum with lymphoid cells and the inhibition of antipeptide serum binding to lymphoid cells by F(ab′)2 fragments of mAbs were performed as described previously,19 with minor modifications. Briefly, 1:20 dilutions of antipeptide mouse sera were mixed with 5 × 105 cells previously preincubated with 50 μL PBS solution containing rabbit IgG (50 μg/mL). After a 30-minute incubation on ice and washings, cells were mixed with an appropriate dilution of FITC-labeled affinity-purified xeno-antibodies to mouse IgG (Fc portion), and cell fluorescence staining was measured using a FACScan cytometer (Becton Dickinson).
In the inhibition assay, rabbit IgG-treated cells were incubated with different concentrations of purified F(ab′)2 fragments of mAbs for 30 minutes on ice. The cells were washed, incubated with antipeptide sera, and the assay was continued as described for the fluorescence binding assay.
CDC. CDC was performed as described,24 with minor modifications. Briefly, 10 μL DTT-treated complement-inactivated antipeptide sera or of PBS solution containing rituximab (500 ng/mL) was added to wells of a round-bottom 96-well plate (Corning Costar, Cambridge, MA) containing B-lymphoid cells (1 × 104/well in 10 μL complete medium). After a 30-minute incubation at 4°C, 50 μL complete medium containing an appropriate dilution of rabbit complement (BAG, Lich, Germany) was added to the mixture and incubation was prolonged for 1 hour at 25°C. Cells were assessed for viability by trypan blue exclusion and counted with a hemocytometer. Lysis was calculated according to the following formula: 100 × ([% viable cells with Abs in the absence of complement - % viable cells with Abs in the presence of complement]/[% viable cells with Abs in the absence of complement]).
In the inhibition by peptide of rituximab-induced CDC, 1 μL PBS solution containing a 10-fold dilution of peptide or control protein (starting concentration 1 mg/mL) was added to 9 μL PBS solution containing rituximab (500 ng/mL). After a 1-hour incubation at 4°C, the mixture was added to wells of a round-bottom 96-well plate (Corning Costar) containing the cells and the assay was continued as described in “CDC.”
Balb/c mouse immunization
Balb/c mouse immunization was performed as described previously,19 except that 10 μg peptide was consistently used for the priming and the 7-, 14-, and 21-day boosting. Sera were harvested on day 28 and every week thereafter up to the eighth week. Sera drawn on day 28 displayed the highest binding titer with the corresponding immunogen and were used for the assay.
Immunoprecipitation experiments
Raji and CEM cells (1 × 108 cells/mL) were washed, pelleted, and lysed on ice for 30 minutes in lysis buffer containing protease inhibitors, as previously described.4 Nuclei and insoluble material were removed by centrifugation at 13 000g for 30 minutes at 4°C. Lysate was then precleared by 2 incubations, each for at least 1 hour at 4°C, with PG-Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ; 30 μL packed resin/mL of lysate). Then, 100 μL lysate solution was added to 10 μL packed PG-Sepharose, which had been previously armed with mAbs or mouse sera. After a 2-hour incubation at 4°C, the beads were extensively washed with lysis buffer. Precipitated proteins were eluted in SDS sample buffer, separated by SDS-PAGE, and transferred to a PVDF membrane. Immune detection was performed as described.4,19
Results
Selection of phage-displayed peptides reactive with rituximab
To identify the cyclic or linear peptides recognized by rituximab, phage clones were isolated by panning each of the 3 PDPLs with rituximab. At each round, phage particles binding to isotypic and allotypic determinants of rituximab were removed by a preadsorption step on the rituximab isotype-matched infliximab. Similarly, protein A and protein G were used alternately during panning to minimize the isolation of phage-displayed peptides reactive with these proteins.
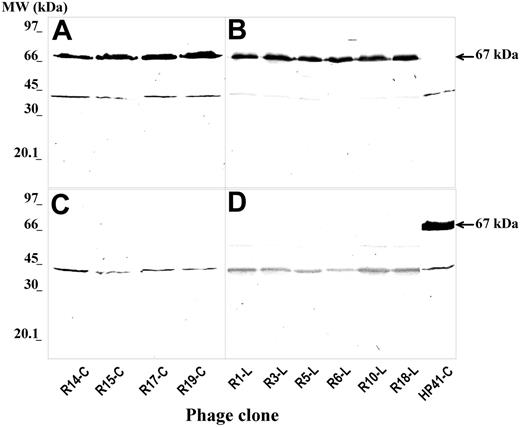
Immunoscreening of 20 randomly selected colonies from each of the 3 PDPLs at the end of the third round of panning showed that 13 (65%; from c7c PDPLs), 9 (45%; from 7-mer PDPLs), and 10 (50%; from 12-mer PDPLs) reacted with rituximab. The binding was specific, since no reactivity with infliximab was detected (Table 1). Specificity of their reactivity was further assessed in Western blot (representative results are shown in Figure 1). Biotinylated rituximab reacted with minor-coat protein III (pIII)-fused peptide of phagotopes (Figure 1A-B). The reactivity was specific since rituximab did not react with the anti-CD4 mAb HP2/6-specific phagotope HP41-C, nor did rituximab-specific phage clones react with either anti-CD4 mAb HP2/6 (Figure 1C-D) or infliximab (data not shown). Rituximab-positive phage clones were selected for sequencing.
Western blot analysis of the specific reactivity of rituximab with phage minor-coat PIII protein-fused peptides (arrows) from the positive ELISA phage clones. Purified phage particles (1 × 1010 pfu's/lane) isolated by biopanning rituximab with c7c (A,C) and 7- or 12-mer (B,D) PDPLs were run onto a 10% polyacrylamide gel under nonreducing conditions and transferred to a PVDF filter. The filter was incubated with biotinylated rituximab (A-B) and the assay was continued as described in “Affinity selection, immunoscreening, Western blot, and sequence analysis.” Binding of rituximab to anti-CD4 mAb HP2/6-specific phage clone (HP41-C) and of biotinylated mAb HP2/6 with rituximab-specific phage particles (C-D) were included as specificity controls. MW indicates molecular weight.
Western blot analysis of the specific reactivity of rituximab with phage minor-coat PIII protein-fused peptides (arrows) from the positive ELISA phage clones. Purified phage particles (1 × 1010 pfu's/lane) isolated by biopanning rituximab with c7c (A,C) and 7- or 12-mer (B,D) PDPLs were run onto a 10% polyacrylamide gel under nonreducing conditions and transferred to a PVDF filter. The filter was incubated with biotinylated rituximab (A-B) and the assay was continued as described in “Affinity selection, immunoscreening, Western blot, and sequence analysis.” Binding of rituximab to anti-CD4 mAb HP2/6-specific phage clone (HP41-C) and of biotinylated mAb HP2/6 with rituximab-specific phage particles (C-D) were included as specificity controls. MW indicates molecular weight.
Mapping of rituximab-specific epitope on CD20 antigen (Ag)
Nucleotide sequence analysis of positive phage clones isolated from c7c and 7- and 12-mer PDPLs identified 11, 1, and 5 distinct sequences, respectively, in the peptides expressed from a total of 13, 9, and 10 rituximab-specific clones isolated from c7c, 7-mer, and 12-mer PDPLs, respectively (Table 1).
Alignment of the deduced amino acid sequences of the inserts from c7c PDPL-derived positive clones resulted in the motif A(S)NPS (Table 1) and this could be aligned with the 170ANPS173 stretch located on the extracellular loop of CD20. N, P, and S were present in all phage-derived peptides, suggesting that they are essential for rituximab-specific epitope expression. Before the motif, alanine was replaced by serine in 6 of the 11 distinct sequences, suggesting that these 2 amino acids can be used interchangeably for CD20 epitope expression.
Alignment of the deduced amino acid sequences of the inserts from the 7- and 12-mer PDPL-derived phage-positive clones resulted in the motif WPxWLE (Table 1). An NCBInr database26 search showed that the motif could not be aligned with any human protein sequence, whereas the reverse ELWxPW could only be aligned with amino acids 156ELWPW161 of ASMLPD (Swiss Prot database nos. Q92485 and Q5T0Y8).
Design and synthesis of cyclic and linear peptides
To determine the contribution of each ANPS-motif amino acid to rituximab-epitope expression compared with amino acids found at the same position on mouse (m) CD20 sequence (170SNSS173), the following cyclic and linear peptides were designed for synthesis (Table 2): Rp15-C < acPYANPSLc > (motif amino acids are in bold), with the insert sequence of phage clone R15-C, bore the motif that perfectly matched the human (h) CD20 sequence 170ANPS173; peptide Rp3-C < acPYSNPSLc >, with the insert sequence of phage clone R3-C, differs from Rp15-C because of the replacement of alanine with serine, which is at position 170 of mCD20; a third cyclic peptide mRp3-C < acPYSNSSLc > was synthesized with the sequence of Rp3-C, except that the motif proline was replaced by serine. This peptide's motif completely matched 170SNSS173 of mCD20. As far as linear peptides are concerned, Rp1-L was synthesized on the basis of the insert sequence expressed by all 7-mer PDPL-derived positive clones, whereas Rp5-L was synthesized on the basis of the insert sequence expressed by 12-mer PDPL-derived positive clone R5-L.
The hCD20 amino acid stretch containing the motif ANPS was used as the template to design and synthesize a linear peptide RpCD20165-184-L (RpCD20-L), which was used as control peptide throughout the experiments.
To test the reactivity of rituximab with ASMLPD, an 11-mer pASMLPD-derived peptide < 153QIAELWKPWLS163 > and rev-pASMLPD < 163SLWPKWLEAIQ153 > with reverse-oriented sequence were synthesized, which included the motif expressed by linear peptides.
Reactivity of peptides with rituximab
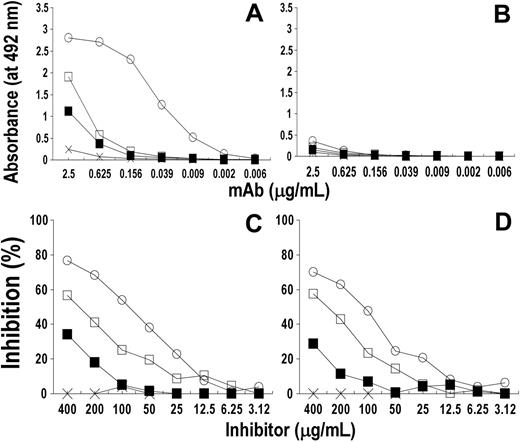
Preliminary experiments showed that rituximab did not bind peptides coated to the plates (data not shown) either because peptides were unable to bind to the plates or because of changes in their conformation due to their binding to the plate. Therefore, peptides were conjugated to a protein carrier, KLH, before testing their reactivity with different concentrations of rituximab. Figure 2A showed that this mAb reacted with the KLH-cyclic peptides Rp15-C and Rp3-C to a similar extent, whereas it did not react (or only slightly) with mRp3-C. Rituximab reacted with the 2 linear KLH peptides, though the reactivity with Rp5-L was higher than that with Rp1-L and with RpCD20-L. Binding was specific since rituximab did not react with KLH-Qp1a nor did rituximab-specific peptides react with the anti-TNF-α infliximab (Figure 2B,D). The data indicate that conjugation to KLH of peptides did not change their antigenic profiles and that the A(S)NPS-motif amino acid proline is the most critical for rituximab binding, since its replacement with serine of the mouse sequence, as in mRp3-C, markedly reduced the epitope expression.
Specificity of the reactivity of rituximab with cyclic and linear peptides following their conjugation to KLH. Ninety-six-well polyvinyl-chloride microtiter plates were incubated with 50 μL of PBS solution containing KLH-cyclic peptides Rp15-C (○), Rp3-C (▵), and mRp3-C (⋄) (A-B) and linear peptides Rp1-L (♦), Rp5-L (▴), RpCD20-L (▪) (C-D) (5 μg/mL) for 12 hours at 4°C. After 2 washings and blockage of free protein-binding sites, a 4-fold serial dilution of rituximab (A,C) or infliximab (negative control; B,D) was added to the plate and their binding to peptide was detected with HRP-conjugated xeno-antibodies to human IgG (Fc portion). Bindings of rituximab to KLH-Qp1a (×) and to KLH (□) were included as negative controls.
Specificity of the reactivity of rituximab with cyclic and linear peptides following their conjugation to KLH. Ninety-six-well polyvinyl-chloride microtiter plates were incubated with 50 μL of PBS solution containing KLH-cyclic peptides Rp15-C (○), Rp3-C (▵), and mRp3-C (⋄) (A-B) and linear peptides Rp1-L (♦), Rp5-L (▴), RpCD20-L (▪) (C-D) (5 μg/mL) for 12 hours at 4°C. After 2 washings and blockage of free protein-binding sites, a 4-fold serial dilution of rituximab (A,C) or infliximab (negative control; B,D) was added to the plate and their binding to peptide was detected with HRP-conjugated xeno-antibodies to human IgG (Fc portion). Bindings of rituximab to KLH-Qp1a (×) and to KLH (□) were included as negative controls.
Mapping the cyclic and linear peptide contact site
To define whether the epitopes recognized by linear and cyclic peptides are distant or spatially related to each other, a cross-blocking assay was performed by testing the binding of rituximab to linear KLH-Rp1, -Rp5, and -RpCD20-L peptides and to the KLH-cyclic peptide Rp15-C in the presence of different concentrations of free peptide. Figure 3 shows that each peptide inhibited the binding of rituximab to the homologous peptide to a similar extent (dashed line). The inhibition was dose dependent and specific, since peptide Qp1a displayed no inhibitory effect. Rp1-L and Rp5-L inhibited the binding of rituximab to Rp15-C and to RpCD20-L, whereas Rp15-C affected rituximab binding to Rp1-L and RpCD20-L. Neither Rp15-C nor RpCD20-L affected the Rp5-L reactivity with rituximab.
The data indicate that the epitope recognized by Rp15-C is spatially related (if not identical) to that recognized by linear peptides and that the lack of inhibition by Rp15-C and RpCD20-L of Rp5-L reactivity with rituximab probably reflects their relative avidity binding for rituximab being lower than that of Rp5-L.
Peptides recognize the rituximab antigen-combining site
To define whether the synthetic peptides recognize the rituximab Ag-combining site, their ability to inhibit rituximab binding to CD20+ human B-lymphoid Raji cells or rituximab-induced CDC was evaluated. As shown in Figure 4A, Rp1-L, Rp5-L, Rp15-C, and, to a lower extent, Rp3-C inhibited rituximab binding. The inhibition was dose dependent and specific since peptide Qp1a did not affect the binding. Rp15-C was an efficient inhibitor, as 72.52 μg/mL was required to inhibit rituximab binding by 50% (50% inhibitory concentration [IC50]), whereas 204.79 μg/mL of Rp3-C was required for IC50. Among linear peptides, Rp5-L was a more efficient inhibitor (IC50, 28.02 μg/mL) than Rp1-L (IC50, 39.02 μg/mL). RpCD20-L, on the other hand, barely inhibited rituximab binding at high concentration, whereas mRp3-C had no effect on the interaction. Rp1-L, Rp5-L, and Rp15-C dose-dependently inhibited the rituximab-induced CDC, though the extent of inhibition was lower than that of rituximab F(ab′)2 (our positive control; Figure 4B). The inhibition was specific since neither Qp1a nor infliximab F(ab′)2 affected the cytotoxicity. The results suggest that the epitope(s) recognized by Rp1-L, Rp5-L, and Rp15-C is within the rituximab Ag-combining site.
Cross-blocking assay to define the spatial relationship between linear and cyclic peptide contact sites. Different concentrations of Rp1-L (♦), Rp5-L (▴), RpCD20-L (▪), and Rp15-C (○) were mixed with an appropriate dilution of purified rituximab (3 μg/mL). After 1-hour incubation at 4°C, the mixture was added to wells of a 96-well microtiter plate previously sensitized with peptide Rp1-L (A), Rp5-L (B), Rp15-C (C), and RpCD20-L (D). Rituximab binding to peptide was then detected with HRP-conjugated xeno-antibodies to human IgG (Fc portion). Binding of rituximab to peptide in the presence of homologous peptide (broken line) and of Qp1a peptide (×) were included as positive and negative controls, respectively. Results are expressed as percentage of binding compared with binding in the absence of inhibitor.
Cross-blocking assay to define the spatial relationship between linear and cyclic peptide contact sites. Different concentrations of Rp1-L (♦), Rp5-L (▴), RpCD20-L (▪), and Rp15-C (○) were mixed with an appropriate dilution of purified rituximab (3 μg/mL). After 1-hour incubation at 4°C, the mixture was added to wells of a 96-well microtiter plate previously sensitized with peptide Rp1-L (A), Rp5-L (B), Rp15-C (C), and RpCD20-L (D). Rituximab binding to peptide was then detected with HRP-conjugated xeno-antibodies to human IgG (Fc portion). Binding of rituximab to peptide in the presence of homologous peptide (broken line) and of Qp1a peptide (×) were included as positive and negative controls, respectively. Results are expressed as percentage of binding compared with binding in the absence of inhibitor.
Inhibition assay to define the spatial relationship between the peptide-specific epitopes and the rituximab antigen-combining site. (A) Fifty microliters of a PBS solution containing 2-fold serial dilutions of Rp1-L (♦), Rp5-L (▴), Rp15-C (○), Rp3-C (▵), mRp3-C (□), and RpCD20-L (▪) was mixed with an equal volume of an appropriate dilution of biotinylated rituximab. After 2-hour incubation at 4 °C, the mixture was added to target Raji cells (7 × 105 cells/well) and their binding to rituximab was detected with HRP-avidin. Binding of rituximab in the presence of unlabeled rituximab (+), infliximab (•), and peptide Qp1a (×) were included as specificity controls. Results are expressed as percentage inhibition of binding compared with binding in the absence of inhibitor. (B) One microliter of PBS solution containing 10-fold serial dilution of Rp1-L (♦), Rp5-L (▴), and Rp15-C (○; starting concentration 1 mg/mL) was mixed with 9 μL PBS solution containing rituximab (500 ng/mL). After 1-hour incubation at 4°C, the mixture was added to Raji cells (1 × 104 cells/well). Following 30-minute incubation at 25°C, an appropriate dilution of rabbit complement was added to each well and the assay was continued as described in “CDC assay.” Lysis mediated by rituximab in the presence of infliximab F(ab′)2 (•) and mAb HC-10-specific peptide Qp1a (□) was included as negative control. Lysis mediated by rituximab in the presence of rituximab F(ab′)2 (+) was used as positive control.
Inhibition assay to define the spatial relationship between the peptide-specific epitopes and the rituximab antigen-combining site. (A) Fifty microliters of a PBS solution containing 2-fold serial dilutions of Rp1-L (♦), Rp5-L (▴), Rp15-C (○), Rp3-C (▵), mRp3-C (□), and RpCD20-L (▪) was mixed with an equal volume of an appropriate dilution of biotinylated rituximab. After 2-hour incubation at 4 °C, the mixture was added to target Raji cells (7 × 105 cells/well) and their binding to rituximab was detected with HRP-avidin. Binding of rituximab in the presence of unlabeled rituximab (+), infliximab (•), and peptide Qp1a (×) were included as specificity controls. Results are expressed as percentage inhibition of binding compared with binding in the absence of inhibitor. (B) One microliter of PBS solution containing 10-fold serial dilution of Rp1-L (♦), Rp5-L (▴), and Rp15-C (○; starting concentration 1 mg/mL) was mixed with 9 μL PBS solution containing rituximab (500 ng/mL). After 1-hour incubation at 4°C, the mixture was added to Raji cells (1 × 104 cells/well). Following 30-minute incubation at 25°C, an appropriate dilution of rabbit complement was added to each well and the assay was continued as described in “CDC assay.” Lysis mediated by rituximab in the presence of infliximab F(ab′)2 (•) and mAb HC-10-specific peptide Qp1a (□) was included as negative control. Lysis mediated by rituximab in the presence of rituximab F(ab′)2 (+) was used as positive control.
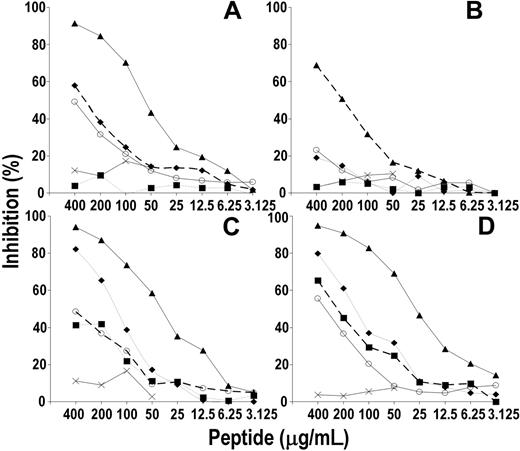
Reactivity profile of a panel of anti-hCD20 mAbs with rituximab-specific peptides
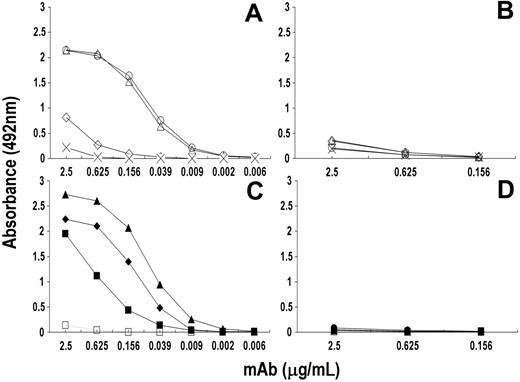
The reactivity profile of rituximab with its paratope-specific peptides was then compared with that of a panel of anti-CD20 mAbs. Figure 5 shows that Rp15-C strongly reacted with rituximab and mAb 1F5 and to a lower extent with mAb AT80; Rp3-C reacted with rituximab and to a lower extent with mAb 1F5, whereas it did not react with AT80. On the other hand, mRp3-C did not react with any of these mAbs, nor did mAb B1, LT20, and 2H7 react with Rp15-C, Rp1-L, and Rp5-L, respectively. The latter 2 linear peptides reacted with rituximab only. As expected, RpCD20-L displayed a broader reactivity pattern in that it reacted with all of the CD20-specific mAbs but CAT13.6. Binding is specific since neither mAb reacted with Qp1a nor did rituximab-specific peptides react with mAb HC-10. The data indicate that rituximab epitope specificity is similar but not identical to that of mAb 1F5 and differs from that of the other anti-CD20 mAbs tested.
Peptides induced antibodies with specificity and effector functions similar to those of rituximab
Two groups of 5 BALB/c mice each were immunized with KLH-conjugated Rp5-L and Rp15-C, respectively, according to the immunization schedule described in “Balb/c mouse immunization.” Three additional mice were immunized with BSA and their sera used as negative control. Sera from all immunized mice reacted with the corresponding immunogen (data not shown). Even so, only sera from 2 of 5 and 1 of 5 Balb/c mice immunized with Rp5-L and Rp15-C, respectively, specifically reacted with Raji and Daudi cells (Figure 6A), whereas they failed to react with CD20- CEM cells (data not shown). Neither anti-Rp5-L nor -Rp15-C sera immunoprecipitated CD20 (data not shown). Sera specificity was assessed in a cross-blocking assay, whereby their reactivity to CD20+ cells was evaluated in the presence of different concentrations of rituximab. Anti-Rp5-L and anti-Rp15-C sera binding was dose-dependently inhibited by preincubation of cells with F(ab′)2 fragments of rituximab, whereas no inhibition was observed when such fragments were replaced with F(ab′)2 fragments of infliximab (representative results are shown in Figure 6B).
Reactivity profile of a panel of anti-CD20 mAbs with the rituximab-specific peptides. Fifty microliters of PBS solution containing anti-CD20 mAb (2.5 μg/mL) was added to wells of a 96-well microtiter plate previously coated with rituximab-specific peptide. After a 4-hour incubation at 25°C, wells were washed and mAb-peptide interaction was detected by the addition of HRP-conjugated xeno-antibodies to the Fc portion of human IgG (to detect rituximab [RIT] and infliximab [INF]) or mouse IgG (to detect the remaining anti-CD20 mAb). Binding of mAb HC-10 to rituximab-specific peptides, and of Qp1a peptide with anti-CD20 mAb, included as negative controls.
Reactivity profile of a panel of anti-CD20 mAbs with the rituximab-specific peptides. Fifty microliters of PBS solution containing anti-CD20 mAb (2.5 μg/mL) was added to wells of a 96-well microtiter plate previously coated with rituximab-specific peptide. After a 4-hour incubation at 25°C, wells were washed and mAb-peptide interaction was detected by the addition of HRP-conjugated xeno-antibodies to the Fc portion of human IgG (to detect rituximab [RIT] and infliximab [INF]) or mouse IgG (to detect the remaining anti-CD20 mAb). Binding of mAb HC-10 to rituximab-specific peptides, and of Qp1a peptide with anti-CD20 mAb, included as negative controls.
Specificity and cytotoxic effect of antibodies elicited in Balb/c mice with Rp5-L and Rp15-C. (A) Fifty microliters of a 1:20 dilution of sera drawn from BALB/c mice immunized with Rp5-L (thick continuous line) and Rp15-C (dashed line) was added to rabbit IgG-treated Daudi cells (5 × 105 cells). After a 30-minute incubation on ice, cells were washed and bound antibodies detected with an appropriate dilution of FITC-labeled affinity-purified xeno-antibodies to mouse IgG (Fc portion). Immunofluorescence was measured using a FACScan cytometer. Binding of anti-CD20 mAb 1F5 was used as positive control (dotted line). The anti-BSA serum-stained profile is indicated (thin continuous line). (B) Fifty microliters of a 1:20 dilution of anti-Rp5-L serum was added to rabbit IgG-treated Daudi cells (5 × 105 cells) previously preincubated for 30 minutes on ice with different concentrations of F(ab′)2 fragments of mAb rituximab (R). The assay was continued as described for panel A. The binding of antipeptide sera to cells preincubated with 100 μg/mL of infliximab (I100) was used as control. The fluorescence profile of cells stained with FITC-labeled probe is indicated (shaded area). (C) Ten microliters of a 2-fold dilution of DTT-treated complement-inactivated anti-Rp5-L (▴), -Rp15-C (○), and -BSA (×negative control) immune sera was added to wells of a round-bottom 96-well plate (Corning Costar) containing Raji cells. After a 30-minute incubation, an appropriate dilution of rabbit complement (BAG, Germany) was added. Cell viability and lysis were calculated as described in “CDC assay.” Lysis obtained by incubation of immune sera in the absence of complement (dashed lines) and of rituximab (500 ng) in the presence (♦) or absence (⋄) of complement were included as specificity controls.
Specificity and cytotoxic effect of antibodies elicited in Balb/c mice with Rp5-L and Rp15-C. (A) Fifty microliters of a 1:20 dilution of sera drawn from BALB/c mice immunized with Rp5-L (thick continuous line) and Rp15-C (dashed line) was added to rabbit IgG-treated Daudi cells (5 × 105 cells). After a 30-minute incubation on ice, cells were washed and bound antibodies detected with an appropriate dilution of FITC-labeled affinity-purified xeno-antibodies to mouse IgG (Fc portion). Immunofluorescence was measured using a FACScan cytometer. Binding of anti-CD20 mAb 1F5 was used as positive control (dotted line). The anti-BSA serum-stained profile is indicated (thin continuous line). (B) Fifty microliters of a 1:20 dilution of anti-Rp5-L serum was added to rabbit IgG-treated Daudi cells (5 × 105 cells) previously preincubated for 30 minutes on ice with different concentrations of F(ab′)2 fragments of mAb rituximab (R). The assay was continued as described for panel A. The binding of antipeptide sera to cells preincubated with 100 μg/mL of infliximab (I100) was used as control. The fluorescence profile of cells stained with FITC-labeled probe is indicated (shaded area). (C) Ten microliters of a 2-fold dilution of DTT-treated complement-inactivated anti-Rp5-L (▴), -Rp15-C (○), and -BSA (×negative control) immune sera was added to wells of a round-bottom 96-well plate (Corning Costar) containing Raji cells. After a 30-minute incubation, an appropriate dilution of rabbit complement (BAG, Germany) was added. Cell viability and lysis were calculated as described in “CDC assay.” Lysis obtained by incubation of immune sera in the absence of complement (dashed lines) and of rituximab (500 ng) in the presence (♦) or absence (⋄) of complement were included as specificity controls.
Furthermore, anti-Rp5-L and anti-Rp15-C sera were cytotoxic if incubated with Raji or Daudi in the presence of complement, whereas no cytotoxicity was detected in the absence of complement or when cells were incubated with BSA immune sera (Figure 6C).
Rituximab reactivity with ASMLPD-derived peptide
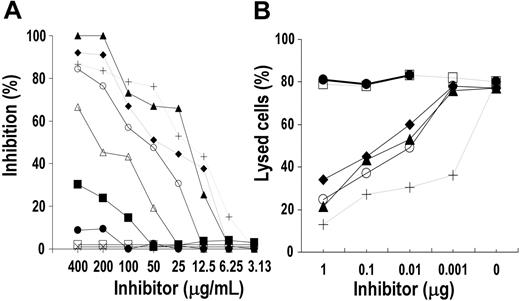
Since ASMLPD is not available in a purified form, the possible interaction of rituximab with this enzyme precursor bearing the reverse-oriented sequence of the motif WPXWLE was assessed by evaluating rituximab reactivity with the ASMLPD-derived 11-mer peptide pASMLPD and with rev-pASMLPD synthesized with the reverse-oriented sequence of pASMLPD (Table 2). Figure 7A-B shows that rituximab specifically reacted dose dependently and to a similar extent with either pASMLPD or rev-pASMLPD, though their relative binding avidity for rituximab was lower than that of Rp15-C. Furthermore Rp15-C specifically affected rituximab's interaction with pASMLPD or rev-pASMLPD (Figure 7C-D). The results indicate a carbon backbone direction-independent reactivity of the motif with rituximab and suggest its possible interaction with ASMLPD.
Discussion
Two different phagotope groups that specifically reacted with rituximab have been identified. Phagotopes of the first group, isolated by the biopanning of c7c PDPL with rituximab, are characterized by the motif A(S)NPS at their cysteine-flanked insert sequence and those of the second group, isolated by biopanning 7- and 12-mer PDPLs with rituximab, by the motif WPxWLE.
NPS is expressed by all c7c-selected positive phage clones, indicating that these amino acids are essential for rituximab binding. Before the motif, 6 and 5 of 11 distinct sequences detected expressed alanine and serine, respectively, indicating that these 2 amino acids are used interchangeably without altering epitope expression. A(S)NPS could be aligned with 170ANPS173 of the extracellular loop of hCD20, indicating that this region is likely to be the contact site of rituximab. It is of interest that this region has a very high homology with mCD20, in that the only difference is the replacement of human A170 and P172 with the chemically similar S170 and S172, respectively. Altogether, these data suggest that replacement of the human P172 with the mouse S172 is the only difference that could account for the lack of rituximab reactivity with mouse CD20. The reactivity analysis of rituximab with Rp15-C, Rp3-C, and mRp3-C strongly supports this conclusion. In fact, both Rp15-C (bearing ANPS) and Rp3-C (bearing SNPS) reacted with rituximab and inhibited its binding to CD20+ human Raji B-lymphoid cells, indicating that the replacement of the motif alanine with serine did not affect or only slightly affected rituximab-specific epitope expression. On the other hand, replacement of the SNPS-motif proline with serine completely abolished the reactivity of mRp3-C (bearing SNSS) with rituximab. A similar, though not identical, conclusion was reached by Polyak and Deans4 in site-directed mutagenesis experiments. These investigators showed that replacement of S170 and S172 of the mouse sequence with the chemically similar A170 and P172 of the human sequence, respectively, was sufficient to reconstitute the rituximab epitope, though at that time this mAb reactivity with mouse construct bearing the replacement of human P172 only was not investigated.
The expression of ANPS on cysteine-constrained peptides only indicated that a secondary structure is required for this epitope to be recognized by rituximab. This would explain the lack of an efficient inhibition by the ANPS-bearing linear peptide RpCD20-L of rituximab binding to CD20 Ag and the decreased reactivity of rituximab with phage clone R15-C following its treatment with 2-ME (data not shown).
Binding and inhibition assays to assess the specificity of rituximab reactivity with ASMLPD-derived peptides. (A-B) Fifty microliters of different concentrations of rituximab (A) and infliximab (B) was added to wells of a 96-well microtiter plate previously coated with pASMLPD (▪) and rev-pASMLPD (□) and incubated for 4 hours at 25°C. Peptide-antibody binding was detected with HRP-xeno-antibodies to human IgG (Fc portion). Binding of mAb to Rp15-C (○) and Qp1a (×) was included as specificity control. (C-D) Different concentrations of pASMLPD (▪), rev-pASMLPD (□), and Rp15-C (○) were mixed with an appropriate dilution of purified rituximab (5 μg/mL). After a 1-hour incubation at 4°C, the mixture was added to wells of a 96-well microtiter plate previously sensitized with peptide pASMLPD (C) and rev-pASMLPD (D). Rituximab-peptide interaction was determined with HRP-conjugated xeno-antibodies to human IgG (Fc portion). Binding of rituximab to peptide in the presence of peptide Qp1a (×) was included as negative control. Results are expressed as percentage of binding compared with binding in the absence of inhibitor.
Binding and inhibition assays to assess the specificity of rituximab reactivity with ASMLPD-derived peptides. (A-B) Fifty microliters of different concentrations of rituximab (A) and infliximab (B) was added to wells of a 96-well microtiter plate previously coated with pASMLPD (▪) and rev-pASMLPD (□) and incubated for 4 hours at 25°C. Peptide-antibody binding was detected with HRP-xeno-antibodies to human IgG (Fc portion). Binding of mAb to Rp15-C (○) and Qp1a (×) was included as specificity control. (C-D) Different concentrations of pASMLPD (▪), rev-pASMLPD (□), and Rp15-C (○) were mixed with an appropriate dilution of purified rituximab (5 μg/mL). After a 1-hour incubation at 4°C, the mixture was added to wells of a 96-well microtiter plate previously sensitized with peptide pASMLPD (C) and rev-pASMLPD (D). Rituximab-peptide interaction was determined with HRP-conjugated xeno-antibodies to human IgG (Fc portion). Binding of rituximab to peptide in the presence of peptide Qp1a (×) was included as negative control. Results are expressed as percentage of binding compared with binding in the absence of inhibitor.
The reactivity analysis of anti-CD20 mAb with cyclic and linear rituximab-specific peptides revealed a rituximab reactivity profile that is unique though similar to that of mAb 1F5, as both mAbs were equally reactive with Rp15-C whereas rituximab only reacted with Rp3-C and with the 3 linear peptides. On the other hand, the fine specificity of mAbs 2H7, LT20, and B1, which did not bind Rp15-C, markedly differ from that of rituximab and 1F5. Thus, the possibility to divide anti-CD20 mAbs into Rp15-C-reactive (rituximab and 1F5) and -nonreactive (mAb B1) groups parallels previous characterization of their in vitro function in that mAbs of the former group (type I), such as mAb 1F5 and rituximab, were found to (a) be equally effective in inducing CDC, (b) display the same level of binding at saturating concentration, and (c) be poor inducers of apoptosis and homotypic adhesion, whereas those belonging to the second group (type II), such as mAb B1, were ineffective in CDC but potent inducers of apoptosis and homotypic adhesion.16,17 Altogether these data are at variance with those obtained by Polyak and Deans,4 whose site-directed mutagenesis study showed that rituximab specificity is very similar to B1 and different from 1F5. The discrepancy may reflect the different methods used, since site-directed mutagenesis, which is a useful approach for epitope mapping studies, may not be powerful enough to define the exact contact site of a given mAb.
Phage clones isolated by biopanning the 7- and 12-mer linear PDPLs with rituximab expressed the motif WPXWLE, which could not be aligned with any portion of the extracellular loop of CD20, though the linear peptides Rp1-L and Rp5-L efficiently inhibited rituximab binding to Raji cells. Their contact site is within the rituximab antigen-combining site and spatially related, if not identical, to that of the cyclic peptides. If this is the case, the lack of sharing of amino acid sequences between the linear peptides and the conformational CD20 epitope paralleled similar observations by Luo et al,27 Wagner et al,28 and Riemer et al.29 These investigators characterized peptides that resembled the original antigen and did not share sequence homology with it. In the first and second case, the peptides resembled the high-molecular-weight melanoma-associated antigen epitope recognized by mAb 763.7427 and by 225.28s,28 respectively, whereas in the last case the synthetic peptide resembled the HER-2 epitope recognized by the mAb trastuzumab.29 Altogether, these findings are not surprising, since peptides have been shown to mimic antigens with a completely different structure, namely the polysaccharide capsule of Cryptococcus neoformans,30 the glycan shield of HIV,31 or the pneumococcal capsular polysaccharide.32
The specific reactivity with CD20+ cells of anti-Rp5-L and -Rp15-C immune sera and their cytotoxic effects similar to those observed with rituximab paralleled our own observation with phagotope R10-L-derived Rp10-L33 and suggest that these peptides could potentially be used to target CD20 in an active immunotherapy setting, once the immunization protocol has been optimized. Anti-CD20 antibodies were, in fact, detected in only 2 of 5 and 1 of 5 mice immunized with Rp5-L or Rp15-C, respectively.
Finally, the possibility that antibodies can recognize amino acid side chains independently of the carbon-backbone direction,34,35 as indicated by the reactivity of rituximab with both pASMLPD and rev-pASMLPD, suggested that binding of rituximab to this enzyme is possible. Whether this interaction contributes to the rapid and transient increase in acid sphingomyelinase activity in raft microdomain13 remains to be determined. As ASMLPD has only recently been identified (Swiss Prot database nos. Q92485 and Q5T0Y8), in vitro production of the recombinant protein and generation of specific mAbs will be essential steps to assess the location of this enzyme and its possible biologic significance.
Prepublished online as Blood First Edition Paper, October 13, 2005; DOI 10.1182/blood-2005-04-1769.
Supported by grant 2004-2005 from Associazione Italiana per la Ricerca sul Cancro (AIRC), Milano, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Mr Vito Iacovizzi for his excellent secretarial assistance.

![Figure 5. Reactivity profile of a panel of anti-CD20 mAbs with the rituximab-specific peptides. Fifty microliters of PBS solution containing anti-CD20 mAb (2.5 μg/mL) was added to wells of a 96-well microtiter plate previously coated with rituximab-specific peptide. After a 4-hour incubation at 25°C, wells were washed and mAb-peptide interaction was detected by the addition of HRP-conjugated xeno-antibodies to the Fc portion of human IgG (to detect rituximab [RIT] and infliximab [INF]) or mouse IgG (to detect the remaining anti-CD20 mAb). Binding of mAb HC-10 to rituximab-specific peptides, and of Qp1a peptide with anti-CD20 mAb, included as negative controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-04-1769/4/m_zh80030690400005.jpeg?Expires=1769583676&Signature=l5s8jCFIuLL2b8XxjkRjNmUs~Ngv8u~r1IAzVpaY1su65CGg93EtdKyMSS7QD7wYXs24AuuHw4KmpS~wV3ef1HjCe03P8PRqxztM9~OTXEHaDU6aBNpyrjrUZtZ8qktq9mY0Ygtz2LLFk1BmUJaVub14lsWLvOOu4DQimM8hOlPXTw7uWGE0N~SbzSoT4XD7igXF9RWD1dNTutbrYYtk3xvh5Yh8v9NTDAUWqILUiTFKe~7BE1woMSm8vR3HZp1Jmip-s7uNsh25iKgOSZzcx3EfBX577yKGbgL7qAA0M~xcnXk7cSNBN3siY9UfmnPF9tbPSFWpSJ9LFlAKTjN--w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)