Abstract
Matrix metalloproteinase-9 (MMP-9) activity is required for inflammatory response, leukocyte recruitment, and tumor invasion. There is increasing evidence suggesting that the P2X7 receptor of mononuclear cells, which is activated by extracellular adenosine triphosphate (ATP), is involved in inflammatory responses. In this study, ATP caused a rapid release of MMP-9 and a moderate decrease in tissue inhibitor of metalloproteinase 1 (TIMP-1) release from human peripheral-blood mononuclear cells (PBMCs) over a 30-minute time course. The release was time- and dose-dependent and dissociated from ATP-induced cell death. BzATP, which is the most potent agonist for the P2X7 receptor, also caused a similar effect at a lower dosage. ATP-induced MMP-9 release was inhibited by the P2X7 receptor antagonists periodate oxidized ATP and KN-62, or by calcium chelators, as well as by a loss-of-function polymorphism in the P2X7 receptor, but not by brefeldin A, monensin, or cycloheximide, or by anti–tumor necrosis factor-α (TNF-α) or anti–interleukin-1β (IL-1β) monoclonal antibodies. Results from purified subsets of PBMCs showed monocytes were the major source for MMP-9 and TIMP-1 release, and ATP remained effective in purified monocyte and T-cell populations. These observations suggest a novel role for P2X7 as a pro-inflammatory receptor involved in rapid MMP-9 release and leukocyte recruitment.
Introduction
Synthesis and secretion of matrix metalloproteinases (MMPs) have been demonstrated for all cells of the immune and hemopoietic lineages as well as fibroblasts and endothelial cells. A wide variety of neoplastic cells also have been shown to secrete MMPs, and these MMPs are considered to participate in the metastatic dissemination of tumors1 and cyclic changes in the endometrium.2 Other studies have shown that MMPs are involved in physiologic processes such as angiogenesis wound repair3 and the inflammatory response.4 Among the members of the MMP family, the gelatinases A and B (MMP-2 and 9) are important, since both can produce proteolytic degradation of type 4 collagen, which is the principal component of basement membrane. Many studies have shown that MMP-2 is secreted constitutively, while secretion of MMP-9 is regulated by inflammatory stimuli that differ according to cell type.5 Like the other MMPs, gelatinases are produced in a latent form containing a prodomain, which requires activation by a cysteine-switch mechanism.6 Both gelatinases form tight noncovalent and stable complexes with tissue inhibitor of metalloproteinases (TIMPs). Thus, pro–MMP-2 (72 kDa) specifically binds TIMP-2,7 whereas pro–MMP-9 (92 kDa) specifically binds TIMP-1, which plays a major role in regulating MMP-9 activity.8 Besides the inhibitory effect of TIMP-1, MMP-9 activity also is regulated at various levels from production to secretion, including transcription of the gene by cytokines such as tumor necrosis factor-α (TNF-α) and interleukin 1β (IL-1β) or by cellular interactions such as cell-cell contact between monocytes and activated T lymphocytes, activation of pro-enzyme by other MMPs,9,10 or by variant proteinases such as lipopolysaccharide (LPS)–associated proteinases.11
The P2X7 receptor is a ligand-gated cation channel activated by extracellular adenosine triphosphate (ATP) and highly expressed on monocytes, macrophages, and lymphocytes. Activation of this receptor by exposure to extracellular ATP opens a cation selective channel that allows Ca2+ and Na+ influx and K+ efflux. The channel dilates within the first minute to a larger pore, followed by a cascade of downstream effects, which include the stimulation of phospholipase D,12,13 the activation of membrane metalloproteases,14,15 extensive membrane blebbing,16 and the stimulation of intracellular caspases, which eventually lead to the apoptotic death of the target cell.17 P2X7 activation also leads to release and maturation of both IL-1β and IL-18 from LPS-activated monocytes and macrophages,18-20 as well as release of TNF-α from rat brain microglia.21 To date, 5 loss-of-function polymorphisms have been described in the P2X7 receptor, including a Glu-496 to Ala polymorphism, which affects receptor assembly22 ; an Ile-568 to Asn polymorphism, which prevents receptor trafficking23 ; an Arg-307 to Gln polymorphism, which abolishes ATP binding24 ; a Thr-357 to Ser polymorphism25 ; and a 5′ intronic splice site polymorphism, which lead to a null allele of the P2RX7 gene.26 There is strong evidence from P2X7 gene-deleted mice suggesting that the P2X7 receptor amplifies the inflammatory response,27 but little is known of the mechanism. This study shows that activation of the P2X7 receptor by ATP augments extracellular MMP-9 activity by rapidly increasing MMP-9 release and, in some subjects, decreasing TIMP-1 release.
Materials and methods
Materials
ATP, 2′,3′-O-(4-benzoyl)benzoyl ATP (BzATP), periodate oxidized ATP (OxATP), phorbol 12-myristate 13-acetate (PMA), brefeldin A (BFA), monensin, cycloheximide, barium chloride, magnesium chloride, d-glucose, bovine serum albumin (BSA), RPMI-1640 medium, gentamicin, bistris, ethylenediaminetetraacetic acid (EDTA), 4-aminophenylmercuric acetate (APMA), ϵ-amino-N-caproic acid, N-dodecyl β-d-maltoside, Brij 35, and Triton X-100 were purchased from Sigma (St Louis, MO). HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), fetal calf serum, and Ficoll-Paque PLUS were from Amersham Biosciences (Uppsala, Sweden). 1-(N,O-bis(5-isoquinoline sulfonyl)N-methyl-l-tyrosyl)-4-phenylpiperazine (KN-62), Ro31-9790, the mini-complete protease inhibitor cocktail, and phenylmethylsulfonyl fluoride (PMSF) plus were from Roche Applied Science (Mannheim, Germany). 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis acetoxymethyl ester (BAPTA-AM) was from Molecular Probes (Eugene, OR). The 10% zymogram gels were from Bio-Rad (Hercules, CA). Mouse anti–human MMP-9 mAb was from Santa Cruz Biotechnology (Santa Cruz, CA), fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgG + IgM Ab was from BD Pharmingen (San Jose, CA). Mouse anti–human TNF-α mAb used to neutralize TNF-α biologic activity28 was from BD Biosciences (San Jose, CA); mouse anti–human IL-1β mAb used to block IL-1β activity (according to manufacturer's datasheet) was from R&D System (Minneapolis, MN). MACS magnetic beads coated with anti-CD14, CD19, CD3, and CD56 mAb were from Miltenyi Biotec (Bergisch Gladbach, Germany). CytoTox 96 nonradioactive cytotoxicity assay kit was from Promega (Madison, WI). Human MMP-9 and MMP-2 Biotrak assay kit were from Amersham Biosciences. Human TIMP-1 and TIMP-2 enzyme-linked immunosorbent assay (ELISA) kits were from Oncogene (Darmstadt, Germany).
Source of human peripheral-blood mononuclear cells
Peripheral blood was collected from nonsmoking donors and diluted with an equal volume of RPMI-1640 medium. Peripheral mononuclear cells were separated by density gradient centrifugation over Ficoll-Hypaque and washed once in RPMI-1640 medium and resuspended in RPMI-1640 medium containing 10% fetal calf serum and 5 μg/mL gentamicin (complete RPMI-1640 medium). Monocytes comprised between 9% and 18% of PBMCs from these healthy subjects. Before each experiment, cells were washed twice and resuspended in either HEPES-buffered NaCl medium (145 mM NaCl, 5 mM KCl, 10 mM HEPES, 5 mM d-glucose, and 1 mg/mL BSA, pH 7.5) or HEPES-buffered KCl medium (150 mM KCl, 10 mM HEPES, 5 mM d-glucose, and 1 mg/mL BSA, pH 7.5) in NoStick Hydrophobic 1.5 mL polypropylene tubes (Scientific Specialties Inc, Lodi, CA). In this study, healthy subjects with wild-type P2X7 receptor do not carry any of the known loss-of-function polymorphisms and have high P2X7 function measured by ATP-induced ethidium uptake. Subjects homozygous for the Glu496Ala polymorphism were identified by direct sequencing as previously described22-24 and showed no P2X7 function, as assayed by ethidium influx measurement, in either lymphocyte or monocyte populations. The study was approved by the Human Research Ethics Committee in Sydney West Area Health Service (05/066). Informed consent was provided according to the Declaration of Helsinki.
Gelatin zymography
Mononuclear cells (2.0 × 107/mL) were incubated in NaCl medium or KCl medium with/without ATP or other reagents at 37°C. Both supernatants and cell pellets were collected by centrifugation at 1200g for 3 minutes and frozen at –30°C for 10 to 35 hours. Before assaying, cell pellets were lysed in lysate buffer containing mini-complete protease inhibitor cocktail (EDTA-free, 1 tablet/7 mL), 50 mM bistris, 750 mM ϵ-amino-N-caproic acid, and 1% N-dodecyl β-d-maltoside, pH 7.0). To detect the pro–MMP-9 activity, 10 μL of sample was mixed with 6 μL2 × Laemmli sample buffer and loaded onto a 10% polyacrylamide gel with 0.1% gelatin (zymogram gel). After 2 hours of electrophoresis under constant voltage of 80 to 120 V, gels were washed 3 × 15 minutes in 2.5% Triton X-100, followed by incubation at 37°C for 48 to 72 hours in developing buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], 0.2 M NaCl, 5 mM CaCl2, 1 μM ZnCl2, 0.02% Brij 35, pH 7.5). Gels were stained with 0.5% Coomassie blue G-250 for 30 minutes and de-stained overnight.
Immunostaining of MMP-9
PBMCs (1 × 107/mL) from subjects with wild-type P2X7 function were incubated in Na medium containing 0.1 mM Ca2+ at 37°C with or without 1 μM KN-62 for 15 minutes, followed by addition of 1 mM ATP or 100 nM phorbol myristate acetate (PMA). Cells were washed twice and resuspended in phosphate-buffered saline (PBS). For surface staining, cells were incubated with 4 μg/mL anti–MMP-9 mAb for 20 minutes and washed twice, followed by staining with FITC-conjugated goat anti–mouse IgG + IgM Ab for 15 minutes. For intracellular staining, cells were fixed in 1% paraformaldehyde for 15 minutes at 4°C, washed twice, and resuspended in PBS containing 0.1% saponin. Cells were incubated with 4 μg/mL anti–MMP-9 mAb for 30 minutes and washed twice, followed by staining with FITC-conjugated goat anti–mouse IgG + IgM Ab for another 30 minutes. Fluorescence intensity was measured by BD FACSCalibur flow cytometry.
Purification of monocytes, T lymphocytes, and NK cells by positive selection
PBMCs (5-10 × 107) from randomly selected healthy subjects were resuspended in NaCl medium and incubated with MACS magnetic beads coated with anti-CD14 mAb for 15 minutes at 4°C. Cells were washed once and loaded onto a MACS LS separation column. CD14+ monocytes were collected, as well as the negative fraction. The same steps were repeated to extract CD3+ T lymphocytes and CD56+ natural killer (NK) cells from the negative fraction. The final negative fraction was collected as B lymphocytes. The CD14+ monocytes had fewer than 15% of CD3+, CD19+, or CD56+ cells; the CD3+ T lymphocytes had fewer than 5% of CD14+, CD19+, or CD56+ cells; CD56+ NK cells had fewer than 5% of CD3+, CD14+, or CD19+ cells, while the B lymphocytes had more than 90% of CD19+ cells. All cell suspensions were counted, washed, and resuspended in NaCl medium with 1 mM CaCl2 at a concentration of 2.0 × 107/mL. Supernatants were collected after 60 minutes, and MMP-9 and TIMP-1 concentrations were determined using ELISA kits.
Purification of monocytes and T lymphocytes by negative selection
PBMCs (5-10 × 107) from healthy subjects with fully functional wild-type P2X7 receptors were resuspended in NaCl medium. MACS magnetic beads coated with anti-CD14, CD19, and CD56 mAb were added. Cells were washed once after 15 minutes of incubation at 4°C. T cells were collected through a MACS LD separation column. For monocyte separation, anti-CD3 MACS beads were used instead of anti-CD14 beads. More than 95% purified T cells were CD3+, and more than 90% purified monocytes were CD14+.
MMP-9 and TIMP-1 ELISA
Human MMP-9 Biotrak assay kit (Amersham), which recognizes the 92-kDa MMP-9 and the complex of 92-kDa MMP-9 with TIMP-1, was used to determine MMP-9 concentration. Human TIMP-1 ELISA kit (Oncogene), which reacts with free TIMP-1 and TIMP-1 complexes with MMPs, was used to determine TIMP-1 concentration. Samples were diluted 1:10 for TIMP-1 assay. ELISAs were performed according to the manufacturer's manuals. All samples were stored at –80°C for 1 to 3 weeks before assay.
Ethidium influx measurement by flow cytometry
PBMCs (2 × 106) prelabeled with appropriate fluorophore-conjugated anti-CD19 (for B cell) or anti-CD14 (for monocyte) mAbs were washed once and resuspended in 1.0 mL HEPES-buffered KCl medium (10 mM HEPES, 150 mM KCl, 5 mM D-glucose, 0.1% BSA, pH 7.5) at 37°C. Cells were analyzed at 1000 events per second on a FACSCalibur flow cytometer (Becton Dickinson) and were gated by forward and side scatter and by cell-type–specific antibodies. All samples were stirred and temperature controlled at 37°C using a Time Zero module (Cytek, Fremont, CA). Ethidium (25 μM) was added, followed 40 seconds later by addition of 1.0 mM ATP. The linear mean channel of fluorescence intensity for each gated subpopulation over successive 5-second intervals was analyzed by WinMDI software (version 2.8; Joseph Trotter, The Scripps Institute, La Jolla, CA) and plotted against time. The area under ethidium uptake curve was calculated as the arbitrary unit of the P2X7 receptor function.
LDH detection
The CytoTox 96 nonradioactive cytotoxicity assay kit (Promega) was used to detect l-lactate dehydrogenase (LDH) in supernatant and cell-pellet lysate. Titration of 1:5000 diluted positive control provided in the kit was used as a standard curve. Assay was performed according to the manufacturer's manual.
Statistics
One-sided paired t test was used in this study.
Results
Variability of ATP-induced MMP-9 release
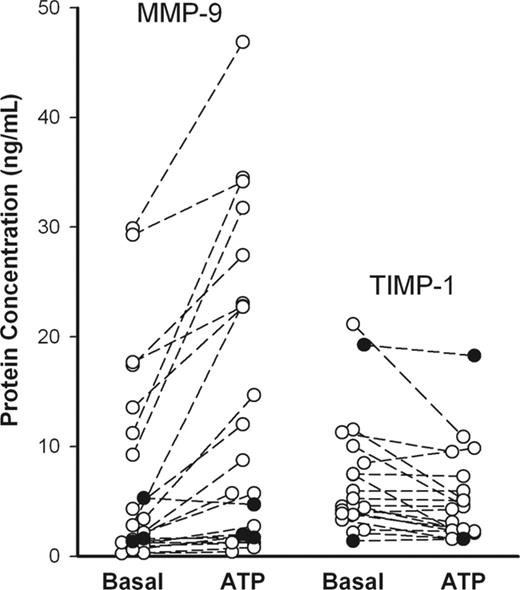
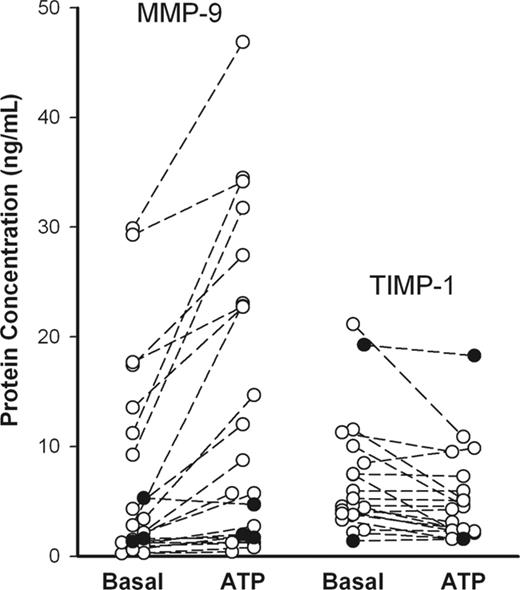
To study ATP effect on MMP-9 and TIMP-1 release, PBMCs from randomly selected healthy subjects (n = 21) were incubated with or without ATP for 30 minutes, and MMP-9 and TIMP-1 concentrations in supernatants were determined using ELISA. Another 3 subjects were studied with genetic absence of P2X7 function due to homozygosity for a loss-of-function polymorphism in the P2RX7 gene. Although the spontaneous releases of MMP-9 and TIMP-1 were quite variable between individuals, an increment in MMP-9 release, which was observed with ATP in 14 of 21 randomly selected subjects, was significant (P = .0002, n = 21). After incubation with ATP for 30 minutes, TIMP-1 release decreased in 9 of the 20 randomly selected subjects and remained at a similar level in the others (Figure 1), although TIMP-1 release increased at longer times of incubation (> 60 minutes) (Figure 2C). In each of the 3 subjects with absent P2X7 function, ATP had a negligible effect on MMP-9 and TIMP-1 release (Figure 1, closed circles). Zymography for subjects with good MMP-9 release showed 3 MMP bands at 220, 130, and 92 kDa in the gel (Figures 2A, 3A, 4A, and S1A, which is available on the Blood website; see the Supplemental Materials link at the top of the online article), with the 220-kDa bands not shown), corresponding to the homodimer of 92-kDa MMP-9, the complex of 92-kDa MMP-9 with 25-kDa lipocalin, and the monomeric MMP-9 in latent form, respectively, as previous described.29 An 84-kDa band showing MMP-9 in active form also was observed when the supernatant was incubated with 2 mM 4-aminophenylmercuric acetate (APMA), an in vitro activator for MMP-9 and MMP-2, for 30 minutes at 37°C before loaded on the gel; however, no MMP band was observed when the MMP inhibitor, 10 mM EDTA or 50 μg/mL Ro31-9790, was added in the developing buffer (data not shown). A 72-kDa MMP-2 band also was found in supernatants from some of the subjects by gelatin zymography; however, ATP failed to alter MMP-2 or TIMP-2 concentration as measured by ELISA after 30 and 60 minutes of incubation (data not shown). To normalize data and reduce variability between individuals, the use of PMA to induce the maximum release of MMP-9 and TIMP-1 was examined.30,31 PBMCs were incubated with various concentrations of PMA (0.1-500 nM) for 15 minutes at 37°C, and MMP-9 activities in supernatant and cell pellet were determined by gelatin zymography, whereas MMP-9 and TIMP-1 concentrations were detected using ELISA kits. PMA (at 5 nM) stimulated maximum MMP-9 and TIMP-1 secretion in 15 minutes (EC50 = 2 nM) when measured either by zymography or by ELISA (Figure S1). Control cells incubated with 10 nM PMA were routinely included in further experiments to obtain maximum MMP-9 release for each subject.
Rapid ATP-induced MMP-9 and TIMP-1 release from mononuclear cells of randomly selected healthy individuals (○) and subjects homozygous for the P2X7 Glu496Ala loss-of-function polymorphism (•). PBMCs (2 × 107/mL) were incubated with or without 1 mM ATP at 37°C in NaCl medium containing 1 mM CaCl2. Supernatants were collected after 30 minutes, and MMP-9 and TIMP-1 concentrations were measured by ELISA. The genotypes of the P2X7 receptor in those randomly selected healthy donors were unknown at the time of assay.
Rapid ATP-induced MMP-9 and TIMP-1 release from mononuclear cells of randomly selected healthy individuals (○) and subjects homozygous for the P2X7 Glu496Ala loss-of-function polymorphism (•). PBMCs (2 × 107/mL) were incubated with or without 1 mM ATP at 37°C in NaCl medium containing 1 mM CaCl2. Supernatants were collected after 30 minutes, and MMP-9 and TIMP-1 concentrations were measured by ELISA. The genotypes of the P2X7 receptor in those randomly selected healthy donors were unknown at the time of assay.
Time course of ATP-induced MMP-9 and TIMP-1 releases
PBMCs from healthy subjects with fully functional wild-type P2X7 receptors were incubated with ATP for 5 to 120 minutes. Increased MMP-9 activity (Figure 2A) and MMP-9 protein level (Figure 2B) appeared in supernatants as soon as 5 minutes after addition of ATP. On average, ATP released 60% (range, 40% to 90%) of the MMP-9 released by PMA (“total”) at 30 to 60 minutes, while only 20% (range, 10% to 60%) of total was spontaneously released over the same time. The increment in MMP-9 due to ATP was significant at 15 minutes and 30 minutes (P value is .003 and .016, respectively, n = 4; Figure 2B). Figure 2C shows that 10% to 45% of total TIMP-1 was released at 30 to 60 minutes, and there was a trend for ATP to inhibit TIMP-1 release. In contrast, quantitation of LDH in the supernatant revealed that ATP-induced cytolysis over the 2 hours of incubation was less than 3% of total, indicating that release of MMP-9 and TIMP-1 was not a result of cell lysis (Figure 2D).
Agonist dose-response for MMP-9 and TIMP-1 release
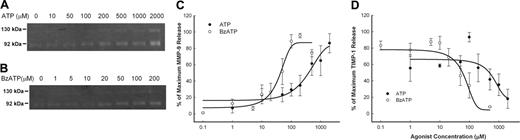
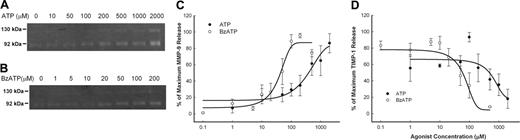
The release of MMP-9 and TIMP-1 from PBMCs was measured over a range of concentrations of ATP as well as BzATP, which is the most potent agonist for the P2X7 receptor. Over a 30-minute incubation, ATP increased MMP-9 release with an EC50 of 300 μM and decreased TIMP-1 release with an IC50 of 500 μM (Figure 3A,C,D), while the more potent BzATP had an EC50 of 50 μM for increasing MMP-9 release and an IC50 of 70 μM for the reduction in TIMP-1 release (Figure 3B-D).
Time-course of ATP-induced MMP-9, TIMP-1, and LDH release. PBMCs (2 × 107/mL) with wild-type P2X7 receptors were incubated with (○) or without (•) 1 mM ATP at 37°C in NaCl medium containing 1 mM CaCl2. Supernatants and cell pellets were collected at different time intervals as indicated. MMP-9 activity in supernatants was detected by gelatin zymography (A). MMP-9 (B) and TIMP-1 (C) concentrations in supernatants were measured using ELISA kits. MMP-9 and TIMP-1 concentrations in supernatant from cells incubated with 100 nM PMA were used as the maximum release (100%). LDH release was measured in both supernatants and cell pellets (D). Results from individuals were normalized and shown as mean ± SEM (n = 4)(B-D). TIMP-1 data are shown for 1 representative experiment of 2.
Time-course of ATP-induced MMP-9, TIMP-1, and LDH release. PBMCs (2 × 107/mL) with wild-type P2X7 receptors were incubated with (○) or without (•) 1 mM ATP at 37°C in NaCl medium containing 1 mM CaCl2. Supernatants and cell pellets were collected at different time intervals as indicated. MMP-9 activity in supernatants was detected by gelatin zymography (A). MMP-9 (B) and TIMP-1 (C) concentrations in supernatants were measured using ELISA kits. MMP-9 and TIMP-1 concentrations in supernatant from cells incubated with 100 nM PMA were used as the maximum release (100%). LDH release was measured in both supernatants and cell pellets (D). Results from individuals were normalized and shown as mean ± SEM (n = 4)(B-D). TIMP-1 data are shown for 1 representative experiment of 2.
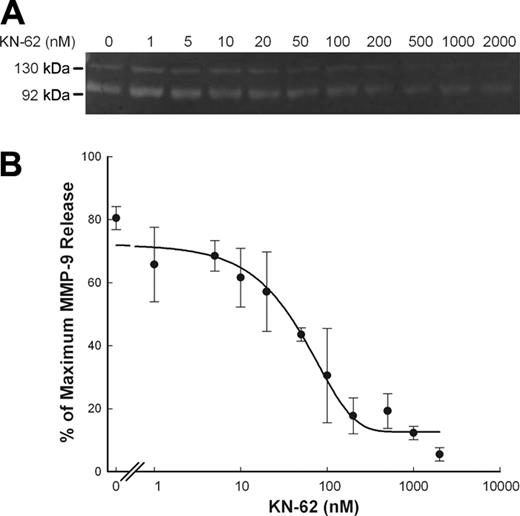
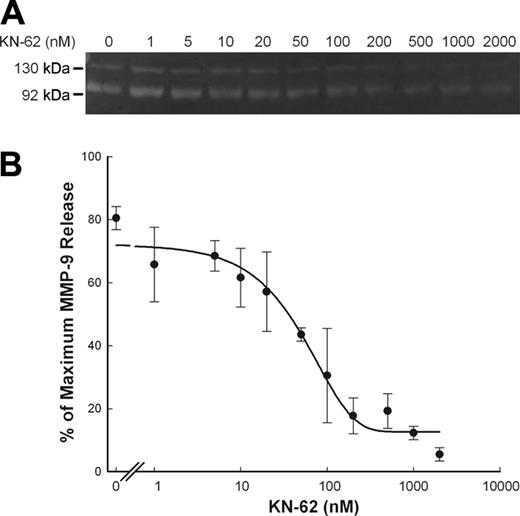
ATP-induced MMP-9 release is inhibited by P2X7 antagonists
KN-62, which is an isoquinolinesulfonamide derivative and a potent and specific inhibitor of the human P2X7 receptor,32 blocked most of the ATP-induced MMP-9 release with an IC50 of 50 nM (Figure 4A,B). Another P2X7 antagonist, OxATP, is widely used as an irreversible antagonist of the P2X7 receptor. Both ATP and BzATP failed to stimulate MMP-9 release in PBMCs pretreated with OxATP (Figure S2). Neither KN-62 nor OxATP affected the spontaneous release of MMP-9.
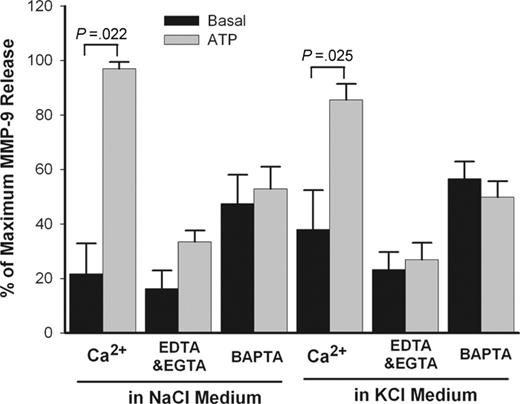
ATP-induced MMP-9 release is Ca2+-dependent
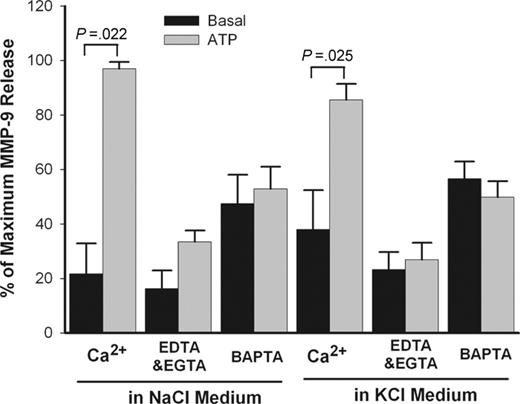
Ca2+ is an important cofactor for a number of secretory events such as P2X7–mediated maturation and secretion of IL-1β from monocytes.33 The effect of Ca2+ on ATP-induced MMP-9 release was studied in PBMC suspensions in which EDTA plus EGTA (ethylene glycol tetraacetic acid) were used to chelate divalent cations in the extracellular NaCl or KCl medium. In separate tubes, cells also were loaded with BAPTA-AM to chelate intracellular Ca2+. Chelation of either extracellular Ca2+ or intracellular Ca2+ produced major inhibitory effects on ATP-induced MMP-9 release in both NaCl and KCl medium (Figure 5). However, no significant differences were found when Ca2+ concentration varied from 10 μM to 1 mM (data not shown). Figure 5 also shows that ATP was equally effective in causing MMP-9 release in NaCl or KCl medium, suggesting that net K+ efflux is not required for MMP-9 release.
Concentration curve of ATP- and BzATP-induced rapid MMP-9 and TIMP-1 release. PBMCs (2 × 107/mL) with wild-type P2X7 receptors were incubated with various amount of ATP (•) or BzATP (○) as indicated at 37°C in NaCl medium containing 1 mM CaCl2 for 30 minutes. Supernatants were collected, and MMP-9 activity (A) was detected by gelatin zymography, and MMP-9 and TIMP-1 concentrations were measured using ELISA kits (B, C). Results from individuals were normalized and shown as mean ± SEM (n = 4 for ATP; n = 5 for BzATP).
Concentration curve of ATP- and BzATP-induced rapid MMP-9 and TIMP-1 release. PBMCs (2 × 107/mL) with wild-type P2X7 receptors were incubated with various amount of ATP (•) or BzATP (○) as indicated at 37°C in NaCl medium containing 1 mM CaCl2 for 30 minutes. Supernatants were collected, and MMP-9 activity (A) was detected by gelatin zymography, and MMP-9 and TIMP-1 concentrations were measured using ELISA kits (B, C). Results from individuals were normalized and shown as mean ± SEM (n = 4 for ATP; n = 5 for BzATP).
MMP-9 and TIMP-1 release from PBMCs is mostly from monocytes
To identify the cell of origin of MMP-9, the different cells of the mononuclear preparation were separated by using antibody-coated magnetic beads. Tables 1 and 2 shows that the basal release of MMP-9 was far greater from the monocytes of PBMCs than for T lymphocytes, whether these cells were separated by either positive or negative selection by magnetic beads. The ATP-stimulated components of MMP-9 release also were far greater for monocytes than for T lymphocytes, although an increment of MMP-9 release could be demonstrated only for negatively selected monocytes, as adhesion of these cells to beads (positive selection) largely abolished the ATP response (Tables 1,2). Table S1 also shows that monocytes released the largest amount of MMP-9 and TIMP-1, followed by B lymphocytes, T lymphocytes, and NK cells. Gelatin zymography also showed monocytes released the highest activity of MMP-9 (data not shown).
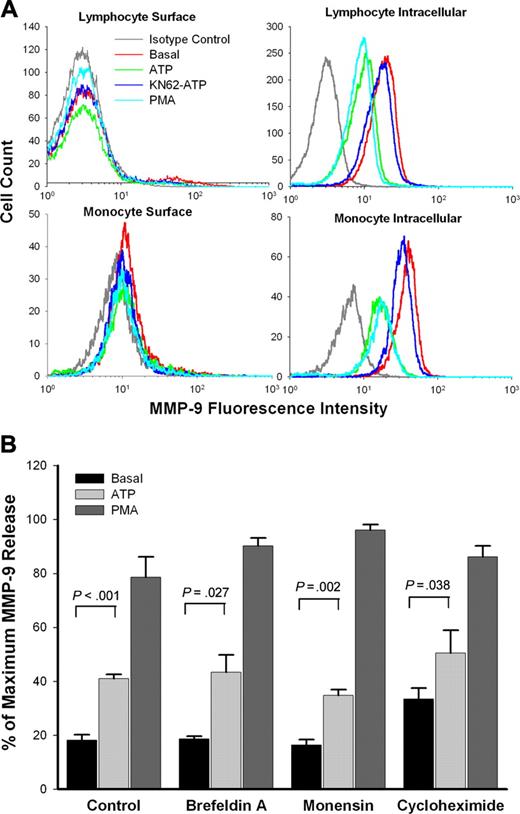
Rapid MMP-9 release is mainly via secretion from intracellular stores
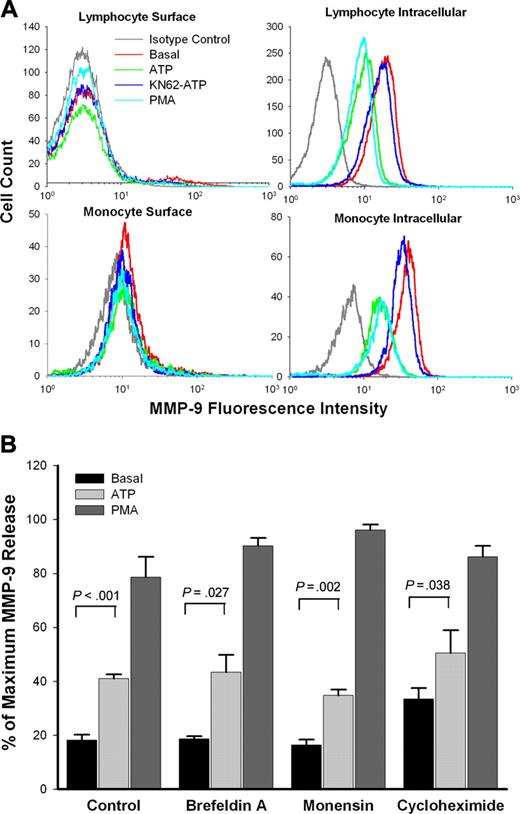
To investigate the source of MMP-9 released to the medium, PBMCs from subjects with wild-type P2X7 receptors were stained with anti–MMP-9 mAb. No MMP-9 was found on the surface of lymphocytes, while monocytes showed weak surface anti–MMP-9 mAb binding (Figure 6A). However, neither ATP nor PMA induced any change in the weak staining on the monocyte surface. In contrast, both lymphocytes and monocytes showed large amounts of intracellular MMP-9, and the amounts were decreased after treatment with either ATP or PMA. Pretreatment of KN-62 abolished this effect of ATP (Figure 6A).
Inhibition of ATP-induced rapid MMP-9 release by KN-62. PBMCs (2 × 107/mL) with wild-type P2X7 receptor were incubated with various amount of KN-62 (up to 2 μM) as indicated at 37°C in NaCl medium containing 1 mM CaCl2 for 15 minutes, followed by addition of 0.8 mM ATP. Supernatants were collected after 30 minutes. MMP-9 activity was detected by gelatin zymography, and MMP-9 concentrations were measured by ELISA. Results from individuals were shown as mean ± SEM (n = 3; panel B).
Inhibition of ATP-induced rapid MMP-9 release by KN-62. PBMCs (2 × 107/mL) with wild-type P2X7 receptor were incubated with various amount of KN-62 (up to 2 μM) as indicated at 37°C in NaCl medium containing 1 mM CaCl2 for 15 minutes, followed by addition of 0.8 mM ATP. Supernatants were collected after 30 minutes. MMP-9 activity was detected by gelatin zymography, and MMP-9 concentrations were measured by ELISA. Results from individuals were shown as mean ± SEM (n = 3; panel B).
Rapid MMP-9 release is via brefeldin A–insensitive pathway
The antiviral antibiotic brefeldin A34 and the sodium ionophore monensin35 are widely used to block protein secretion. Figure 6B shows that the secretion of MMP-9 induced by both ATP and PMA was unaffected by pretreatment of PBMCs with brefeldin A or monensin. Cycloheximide, a widely used inhibitor of protein synthesis,36 also showed no inhibitory effect on ATP- or PMA-induced rapid MMP-9 release over a 30-minute incubation (Figure 6B).
ATP-induced MMP-9 release is independent of TNF-α or IL-1β pathways
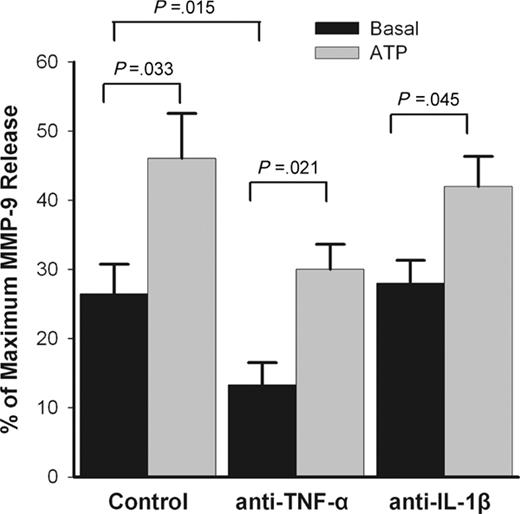
The pro-inflammatory cytokines IL-1β and, especially, TNF-α have been identified as major stimuli for MMP-9 secretion from monocytes and macrophages.37-39 Since monocytes account for most MMP-9 and TIMP-1 production from PBMCs, it is possible that ATP-induced MMP-9 release from mononuclear cells is mediated by a pathway involving either of these 2 proinflammatory cytokines or by some interaction between monocytes and activated T lymphocytes.40 To study the role of these 2 cytokines in ATP-induced MMP-9 release, monocytes and T lymphocytes were first purified from PBMCs by negative selection using magnetic beads. Incubation of each negatively purified subset with ATP still produced an increment of MMP-9 release (Tables 1, 2), suggesting that interaction of these 2 cell types is not essential for ATP-induced MMP-9 release. Further, the addition of blocking monoclonal antibodies to neutralize the biologic activity of TNF-α or IL-1β failed to prevent ATP-induced MMP-9 release (Figure 7). However, the spontaneous release of MMP-9 was significantly reduced by the anti–TNF-α blocking antibody (P = .015, n = 4) (Figure 7), indicating that TNF-α is a major stimulus for the spontaneous MMP-9 release from monocytes, as previously described.36 Activated monocytes that attach to the test tubes also may contribute to the spontaneous MMP-9 release, as a small proportion of monocytes (< 5%) were found attached to the test tubes in control conditions.
Effect of potassium and calcium on ATP-induced rapid MMP-9 release. PBMCs (2 × 107/mL) with wild-type P2X7 receptor were pretreated with or without 50 μM BAPTA-AM for 30 minutes in NaCl medium and washed once. Cells then were incubated with (gray bar) or without (black bar) 1 mM ATP at 37°C in NaCl or KCl medium containing 1 mM CaCl2 or 50 μM EDTA and 50 μM EGTA. Supernatants were collected after 30 minutes. MMP-9 concentrations were measured by ELISA. Results from individuals were normalized and shown as mean ± SEM (n = 3).
Effect of potassium and calcium on ATP-induced rapid MMP-9 release. PBMCs (2 × 107/mL) with wild-type P2X7 receptor were pretreated with or without 50 μM BAPTA-AM for 30 minutes in NaCl medium and washed once. Cells then were incubated with (gray bar) or without (black bar) 1 mM ATP at 37°C in NaCl or KCl medium containing 1 mM CaCl2 or 50 μM EDTA and 50 μM EGTA. Supernatants were collected after 30 minutes. MMP-9 concentrations were measured by ELISA. Results from individuals were normalized and shown as mean ± SEM (n = 3).
Discussion
There is increasing evidence that the P2X7 receptor on cells of monocytic or lymphoid origin participates in the inflammatory response. P2X7 knockout mice show reduced IL-1β release and absence of IL-1β maturation in response to ATP stimulation41 and less severe disease outcomes compared to wild-type mice in anticollagen antibody–induced arthritis, a model of inflammatory joint disease.27 Our data show that the P2X7 receptor also plays a role in rapid release of MMP-9 from monocytes, which is required in the early phase of the inflammatory response, as these cells migrate through basement membrane into the inflammatory focus. Most previous studies have measured the appearance of MMP-9 in supernatants over long time periods (12 to 48 hours) when both production and secretion have to be taken into consideration. However, the short time frame (< 60 minutes) used in the present study may be more relevant to the early events in inflammation, since it has been shown that monocytes can be recruited into inflammatory sites in just 2 to 3 hours, although the numbers steadily increase over 24 hours.42,43 In this short time period of 30 minutes, ATP increased MMP-9 release and decreased TIMP-1 release in a time- and dose-dependent manner (Figures 2,3). This ATP-induced MMP-9 release was inhibited by the P2X7 receptor antagonists KN62 and OxATP (Figures 4, S2), and ATP also failed to induce MMP-9 release from PBMCs of subjects who were homozygous for the P2X7 loss-of-function polymorphism (Figure 1), which abolishes ATP-induced entry of Ca2+ into PBMCs.22 Furthermore, Figure 7 and Tables 1 and 2 show that ATP-induced MMP-9 release is independent of TNF-α– or IL-1β–stimulated MMP-9 release and that the ATP-induced release does not require the interaction of T lymphocytes with monocytes. These observations suggest a novel role for P2X7 as a pro-inflammatory receptor mediating rapid MMP-9 release from monocytes as they migrate into an inflammatory focus.
Pathway for ATP-induced rapid MMP-9 release. (A) PBMCs (1 × 107/mL) were pretreated with or without ATP, or with KN62 plus ATP or PMA as indicated in “Materials and methods.” Fresh cells (surface staining) or fixed cells (intracellular staining) were stained with anti–MMP-9 mAb and FITC-conjugated secondary Ab. The lymphocyte or monocyte populations were identified and gated by forward and side scatter. Flow cytometry histograms show representative data from 1 of 5 subjects with wild-type P2X7 receptors. (B) PBMCs (1 × 107/mL) with wild-type P2X7 receptors were pretreated with or without brefeldin A (10 μg/mL), monensin (5 μg/mL), or cycloheximide (50 μg/mL) for 60 minutes in NaCl medium with 0.1 mM CaCl2 and washed once. Cells then were resuspended at 2 × 107/mL in the same medium with the same concentration of inhibitors, followed by incubation with or without 1 mM ATP (gray bar) or 100 nM PMA (dark gray bar) at 37°C for 30 minutes. MMP-9 concentrations in supernatant were measured by ELISA. Results from individuals were normalized and shown as mean ± SEM (n = 4).
Pathway for ATP-induced rapid MMP-9 release. (A) PBMCs (1 × 107/mL) were pretreated with or without ATP, or with KN62 plus ATP or PMA as indicated in “Materials and methods.” Fresh cells (surface staining) or fixed cells (intracellular staining) were stained with anti–MMP-9 mAb and FITC-conjugated secondary Ab. The lymphocyte or monocyte populations were identified and gated by forward and side scatter. Flow cytometry histograms show representative data from 1 of 5 subjects with wild-type P2X7 receptors. (B) PBMCs (1 × 107/mL) with wild-type P2X7 receptors were pretreated with or without brefeldin A (10 μg/mL), monensin (5 μg/mL), or cycloheximide (50 μg/mL) for 60 minutes in NaCl medium with 0.1 mM CaCl2 and washed once. Cells then were resuspended at 2 × 107/mL in the same medium with the same concentration of inhibitors, followed by incubation with or without 1 mM ATP (gray bar) or 100 nM PMA (dark gray bar) at 37°C for 30 minutes. MMP-9 concentrations in supernatant were measured by ELISA. Results from individuals were normalized and shown as mean ± SEM (n = 4).
Our previous studies have shown that there is wide variation of P2X7 function between individuals,44 largely due to allelic variations in the P2RX7 gene.22-26 In this study, the increments of MMP-9 release after ATP stimulation also varied in randomly selected subjects, and this genetic influence plays an important part. However, other factors, such as spontaneous MMP-9 release and intracellular MMP-9 protein level, also may affect ATP-induced MMP-9 release. Figure 2 shows that spontaneous MMP-9 release increases progressively at longer times of incubation (> 60 minutes). Figure 7 suggests that cytokines, such as TNF-α, may play a major role in spontaneous MMP-9 release as anti–TNF-α mAb significantly reduced spontaneous MMP-9 release. Since monocytes make the major contribution to MMP-9 release (Tables 1, 2, and S1), the percentage of monocytes in PBMCs may be another important factor influencing ATP-induced MMP-9 release. Thus, all of these factors contribute to the considerable variability in MMP-9 release shown in Figure 1.
The P2X7 receptor also has been shown to mediate release and maturation of the pro-inflammatory cytokines IL-1β and IL-18 in LPS-activated monocytes and macrophages.18-20,45 The time course for the ATP-induced release of MMP-9 (92 kDa) in this study (Figure 2) was similar to that reported for pro–IL-1β (31 kDa).18 The release of both molecules is Ca2+ dependent,33 and both are released in a latent form requiring proteolytic processing to become active. Moreover, the concentrations of ATP required to induce MMP-9 release (EC50 = 300 μM) is similar to those required for IL-1β release and maturation.18 However, maturation of IL-1β requires a large K+ efflux from the cell either via the P2X7 pathway or via the K+ ionophore nigericin,19 while MMP-9 precursor is activated in vivo by other members of the MMP family such as MMP-3 (stromelysin)10 or by variant proteinases such as LPS-associated proteinases.11 Furthermore, LPS activation of monocytes is required for ATP-induced IL-1β or IL-18 release but not for ATP-induced MMP-9 release, making the latter applicable to leukocyte recruitment to an inflammatory focus in the absence of gram-negative bacteria.
Blocking ATP-induced rapid MMP-9 release with antibodies. PBMCs (2 × 107/mL) were incubated 5 minutes with or without anti–hu-TNF-α mAb (1:10 dilution) or anti–IL-1β mAb (2 μg/mL) in NaCl medium containing 0.1 mM CaCl2, followed by addition of 1 mM ATP (gray bar). Supernatants were collected after 30 minutes. MMP-9 concentrations were measured by ELISA. Results from individuals were normalized and shown as mean ± SEM (n = 4).
Blocking ATP-induced rapid MMP-9 release with antibodies. PBMCs (2 × 107/mL) were incubated 5 minutes with or without anti–hu-TNF-α mAb (1:10 dilution) or anti–IL-1β mAb (2 μg/mL) in NaCl medium containing 0.1 mM CaCl2, followed by addition of 1 mM ATP (gray bar). Supernatants were collected after 30 minutes. MMP-9 concentrations were measured by ELISA. Results from individuals were normalized and shown as mean ± SEM (n = 4).
Although MMP-9 is not a membrane protein, it has been shown that MMP-9 does bind to a number of surface adhesion molecules and receptors including αMβ2 integrin (Mac-1),46 intercellular adhesion molecule-1 (ICAM-1),47 and Ku heterodimer (Ku70/Ku80),48 which are expressed on the surface of monocytes. It is possible that MMP-9 is rapidly released from the cell surface together with its docking molecules, which are shed by ATP or PMA. However, our results did not support this hypothesis, as ATP or PMA failed to shed MMP-9 on monocyte surface. Instead, large amounts of intracellular MMP-9 were found in monocytes as well as in lymphocytes. ATP reduced the MMP-9 intracellular pool, and this effect was abolished by pretreatment of KN62, suggesting that ATP-induced MMP-9 release is due to secretion of the intracellular MMP-9 store, via activation of the P2X7 receptor.
MMP-9 (92 kDa) has a proposed leading single sequence in its first 19 residues, and it is well-known that long-term (> 7 hours) MMP-9 secretion requires protein synthesis36 and involves the regular protein transport pathway from the endoplasmic reticulum to the Golgi apparatus.49,50 However, in this study, both brefeldin A and monensin failed to block the rapid MMP-9 release induced by ATP or PMA over 30 minutes, suggesting that rapid MMP-9 release may occur via granule secretion, similar to membrane type 1 matrix metalloproteinase (MT1-MMP), which rapidly traffics to the plasma membrane via an unconventional brefeldin A–resistant pathway.51 In support of this concept, it has been shown that B lymphocytes contain MMP-9 in a granular distribution.52 Cycloheximide also failed to block rapid MMP-9 release in this study, in line with a previous report that protein synthesis is not required for rapid MMP-9 release from neutrophils.30
Among the 4 subsets of mononuclear cells, monocytes showed the highest spontaneous release of MMP-9, which may assist the emigration of this cell type from blood into the tissues of the reticulo-endothelial system. Previous studies have shown that monocytes are the first leukocyte type found in an inflammatory site, with cells appearing within 2 to 3 hours.42,43 Elevated concentrations of ATP and its metabolites are found within the inflammatory focus from the breakdown of cellular integrity, and this ATP may help the recruitment of monocytes to the lesion by secretion of MMP-9 and digestion of the extracellular matrix. However, the full biologic significance of the rapid MMP-9 secretion from monocytes remains to be established.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2005-07-2994.
Supported by the National Health and Medical Research Council, the Cure Cancer Australia Foundation, the Leukemia Foundation of Australia, and a Sesqui Fellowship from the University of Sydney (B.J.G.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Steven Fuller for recruitment of healthy subjects, Ms Kristen Skarratt for identification of P2X7 genotype, Dr Ronald Sluyter and A/Prof Lois A. Salamonsen for critical review and discussion, Dr Jin Zhang for advice on gelatin zymography technique, and Ms Jennifer Georgiou for separation of peripheral-blood mononuclear cells from some healthy subjects.