Abstract
T-cell large granular lymphocytic leukemia (T-LGL) is characterized by chronic clonal lymphoproliferation of cytotoxic T lymphocytes (CTLs). Despite exhibiting phenotypic properties of antigen-activated cells, including expression of Fas and FasL, T-LGL cells accumulate and demonstrate resistance to apoptosis. We propose that increased activity of a prosurvival signaling pathway in T-LGL is responsible for attenuated apoptosis in T-LGL. Given the importance of the phosphatidylinositol-3 kinase (PI3K)–AKT pathway in regulating the balance between survival and apoptosis, we analyzed AKT activity in T-LGL cells. Compared with resting CTLs from healthy donors, patients' T-LGL cells showed higher levels of phosphorylated AKT. We demonstrate that phospho-AKT induction is dependent on the upstream activity of a Src family kinase. Since the PI3K-AKT pathway can antagonize the ability of Fas to initiate apoptosis, we hypothesized that inhibition of PI3K would lead to reacquisition of Fas sensitivity in T-LGL. Inhibition of the PI3K-AKT pathway alone led to brisk spontaneous apoptosis of T-LGL. These results suggest that T-LGL pathogenesis is dependent on activity of the PI3K-AKT pathway, without which the leukemic cells will begin to undergo spontaneous apoptosis. We propose that novel therapeutics inhibiting the PI3K-AKT axis may provide effective treatment for T-LGL.
Introduction
T-cell large granular lymphocytic leukemia (T-LGL) is a disease caused by clonal expansion of CD3+CD8+ cytotoxic T lymphocytes (CTLs). Most abnormal cells are terminally differentiated effector CTLs, but it is likely that a fraction of corresponding memory cells exists that replenishes the continuous supply of malignant CTLs.1 Under normal physiologic conditions, CTLs will expand in the course of an immune challenge and then undergo homeostatic apoptosis to prevent untoward reactions directed against the host. According to one hypothesis about the genesis of T-LGL, it is possible that after an initially normal immune response this homeostatic mechanism goes awry, and the T-LGL clone accumulates.2 The most common complications of T-LGL are lineage-restricted cytopenias, such as neutropenia or pure red cell aplasia. They appear to represent a paraneoplastic syndrome due to the cytokines released and/or direct killing by the T-LGL clone equipped with a specific T-cell receptor.3-5
While the pathogenesis of T-LGL remains unknown, lack of homeostatic apoptosis is a remarkable feature of this disease since the clonal CTLs abundantly express Fas and Fas ligand (FasL),2 the most effective pathway for T lymphocyte reduction following resolution of an immune response.6-10 One potential explanation for this finding is that activation of an opposing survival pathway in T-LGL prevents efficient Fas signaling, thus attenuating apoptotic signals. Given the relatively benign clinical course for most cases of T-LGL, we postulated that its likely cause is aberrant activation of a prosurvival signal transduction pathway, possibly mediated via a cytokine receptor. This mechanism would alter the normal balance between survival and apoptosis pathways and explain why T-LGL lymphoproliferation is not entirely autonomous.
A recent report has revealed that T-LGL exhibit constitutive production of the proinflammatory cytokines RANTES (regulated on activation, normal T expressed and secreted), macrophage inflammatory protein 1β (MIP-1β), and interleukin-18 (IL-18).3 Notably, all 3 cytokines have been shown to induce the phosphatidylinositol-3 kinase (PI3K) pathway.11-14 PI3K directs one of the most widely studied prosurvival networks, influencing a wide range of cellular functions, including energy metabolism, cell-cycle progression, and apoptosis. Furthermore, perturbations in the regulation of the PI3K pathway have been described in various neoplasms (reviewed in Vivanco and Sawyers15 ). In particular, PI3K is integral to the malignant process initiated by the Bcr-Abl fusion protein in chronic myelogenous leukemia and also is crucial to survival of chronic B lymphocytic leukemia cells.16-20
We hypothesized that T-LGL exhibit increased PI3K activity, and this may be a key factor in the ability of T-LGL to remain mute to Fas engagement and avoid apoptosis. PI3K pathway activity was analyzed in peripheral blood mononuclear cells (PBMCs) and purified CTLs from healthy donors and patients with T-LGL. The relevance of this pathway to T-LGL pathophysiology was interrogated with the use of pharmacologic inhibitors. We propose that aberrant activation of the PI3K pathway in T-LGL is related to the escape from homeostatic apoptosis and presents a novel therapeutic target for this disease.
Patients, materials, and methods
Patients and controls
Peripheral blood was obtained from healthy volunteers and patients with T-LGL after informed consent was obtained according to protocols approved by the Institutional Review Board of the Cleveland Clinic Foundation (Cleveland, OH). The original diagnosis of LGL leukemia was established by clinical and laboratory parameters as suggested by Berliner et al,21 Semenzato et al,22 and Herling et al.23 Clinical description of the patients and their clonal CTL is provided in Table 1.
Cell separation
Peripheral blood mononuclear cells were isolated by density gradient centrifugation (Mediatech, Herndon, VA). In some experiments purified CD8 cells were used. To isolate CD8 T cells, peripheral blood was incubated with RosetteSep CD8 (Stem Cell Technologies, Vancouver, BC, Canada) for 20 minutes at room temperature (RT) with gentle mixing, followed by density gradient centrifugation, per manufacturer's instructions. This routinely yielded CD8 T cells of 90% or higher purity as determined by flow cytometry (data not shown).
Cell culture and lysates
PBMCs or enriched T-cell populations were resuspended in RPMI-1640 at 5 × 107 cells/mL in the presence or absence of 50 μM LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one), 10 μM U0126 (1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene; Cell Signaling Technology, Beverly, MA), or 10 μM PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; Calbiochem, San Diego, CA) as indicated in the text. Where indicated, cells were stimulated with recombinant human IL-2 (R&D Systems, Minneapolis, MN), or OKT3 (Ortho, Raritan, NJ). Cells were then incubated at 37°C for 15 minutes, followed by immediate addition of an equal volume of 2 × sodium dodecyl sulfate (SDS) lysis buffer (0.125 M Tris-HCl, 20% glycerol, and 4% SDS) and boiled.
Western blotting
Proteins were separated by SDS–polyacrylamide gel electrophoresis (PAGE) on 10% gels under reducing conditions and transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA) in a transfer buffer consisting of 20 mM Tris-HCl, 150 mM glycine, and 20% methanol. Membranes were incubated 1 hour at RT in blocking buffer (Tris buffered saline with 0.1% Tween-20 [TBS-T], 5% nonfat milk). Primary and secondary Abs were diluted 1:1000 in blocking buffer and incubated with the membranes for 1 hour at RT, with washes in TBS-T in between. Detection of horseradish peroxidase (HRP)–conjugated antibodies (Abs) was performed using SuperSignal West Pico (Pierce, Rockford, IL). Chemiluminescence of all membranes was detected using Hyperfilm ECL (Amersham, Piscataway, NJ). The following rabbit polyclonal antibodies were used: phospho-AKT (S473), phospho-Src family kinase (Y419), phospho-p44/42 ERK (T202/Y204), phospho-GSK-3β (S9), and AKT were from Cell Signaling Technology (Beverly, MA). GAPDH was from Trevigen (Gaithersburg, MD).
Apoptosis assay
PBMCs (1 × 106/mL) were aliquoted in 12-well tissue-culture plates in complete medium (RPMI-1640, 10% fetal calf serum [FCS]) in the presence or absence of 50 μM LY294002 and/or 1 μg/mL anti-Fas CH11 (Upstate Biotechnology, Lake Placid, NY), as indicated. Cells were then incubated overnight at 37°C. After 20 hours, cells were harvested and stained with either anti-CD8–PC5 or anti-Vβ2–PE (Beckman-Coulter, Fullerton, CA) and annexin-FITC (BD Biosciences, San Jose, CA) per manufacturer's instructions. Data were acquired on a Coulter Epics XL-MCL flow cytometer (Beckman Coulter, Miami, FL) and analyzed with WinMDI 2.8 (Joseph Trotter, Scripps Research Institute, La Jolla, CA). Micrographs were captured on an Olympus BX50 microscope (Olympus, Tokyo, Japan) with a Spot Insight QE digital camera using Spot Basic software version 4.0.5 (Spot Diagnostic Instruments, Sterling Heights, MI). Low-power magnification was performed using a 20 ×/0.4 NA objective, final magnification 200 ×. High-power magnification was performed using a 100 ×/1.25 NA oil objective, final magnification 1000 ×. Images are shown as captured, without further processing.
Results
PI3K activity in T-LGL
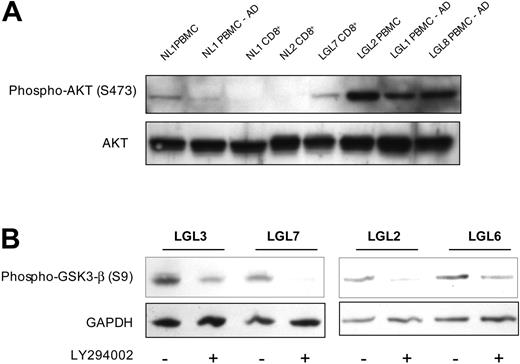
Defective signaling along survival pathways may be involved in the pathogenesis of the clonal lymphoproliferation in T-LGL. To assess the potential role of the PI3K pathway in T-LGL, we compared PBMCs from patients with T-LGL to healthy-donor PBMCs (Figure 1). For analysis, we selected patients with a very high proportion of clonal CTLs (Table 1), assuring that our observations are relevant to the pathologic clone. However, since the mononuclear cells comprise a mixed population, including monocytes that may have higher resting levels of PI3K activity,24,25 we also compared healthy-donor freshly isolated PBMCs versus PBMCs that were depleted of adherent cells (Figure 1A, lanes 1 and 2). In normal PBMCs comprising a mix of T cells, B cells, natural killer (NK) cells, and monocytes there was a detectable PI3K pathway activity as measured by the presence of phosphorylated AKT (a downstream target). However, when the adherent cells were removed, there was a marked loss of AKT phosphorylation, suggesting that the remaining nonadherent cells, including T cells, do not have appreciable PI3K activity. Contrary to PBMCs from healthy donors, patients with T-LGL had readily detectable AKT phosphorylation in both freshly isolated and adherent-depleted PBMCs (Figure 1A, lanes 6-8). To further qualify these results, we specifically isolated CD8 T cells (≥ 90% enrichment) from healthy individuals and patients with T-LGL for analysis. Within the enriched CD8 cell populations from healthy donors we detected very little to no AKT phosphorylation, while the purified clonal T-LGL cells had elevated phospho-AKT (Figure 1A, lanes 3-5). In each patient with T-LGL who we have studied thus far, elevated levels of phospho-AKT have been observed.
PI3K-AKT pathway activity in T-LGL cells. (A) Lysates of purified CD8 T cells or PBMCs before and after adherent cell depletion, as indicated, from healthy donors and patients with T-LGL were prepared by direct addition of 2 × SDS sample buffer after a brief 15-minute incubation at 37°C and separated on a 10% SDS-PAGE gel. Following transfer to nitrocellulose membrane, samples were sequentially analyzed for phosphorylated AKT (S473) and total AKT protein via immunoblotting. Cell equivalents (3 × 105) were loaded in all lanes. (B) PBMCs from 4 patients with T-LGL were incubated at 37°C for 15 minutes in the presence or absence of LY294002 (50 μM), followed by immediate addition of 2 × SDS sample buffer. Proteins were separated on an SDS-PAGE gel as in panel A, transferred to nitrocellulose, and immunoblotted for phosphorylated GSK-3β (S9). GAPDH levels were probed to indicate consistency of protein loading.
PI3K-AKT pathway activity in T-LGL cells. (A) Lysates of purified CD8 T cells or PBMCs before and after adherent cell depletion, as indicated, from healthy donors and patients with T-LGL were prepared by direct addition of 2 × SDS sample buffer after a brief 15-minute incubation at 37°C and separated on a 10% SDS-PAGE gel. Following transfer to nitrocellulose membrane, samples were sequentially analyzed for phosphorylated AKT (S473) and total AKT protein via immunoblotting. Cell equivalents (3 × 105) were loaded in all lanes. (B) PBMCs from 4 patients with T-LGL were incubated at 37°C for 15 minutes in the presence or absence of LY294002 (50 μM), followed by immediate addition of 2 × SDS sample buffer. Proteins were separated on an SDS-PAGE gel as in panel A, transferred to nitrocellulose, and immunoblotted for phosphorylated GSK-3β (S9). GAPDH levels were probed to indicate consistency of protein loading.
To further substantiate increased activity of the PI3K pathway in T-LGL, we examined a downstream target of AKT for evidence of phosphorylation. Glycogen synthase kinase-3 (GSK3) is inactivated by AKT-mediated phosphorylation at serine 9, thus relieving GSK3, inhibition of multiple targets regulating transcription, proliferation, and apoptosis.15,27 PBMCs from patients with T-LGL demonstrate phosphorylated GSK3, (Figure 1B, lanes 1, 3, 5, and 7), consistent with activation of the PI3K-AKT pathway. Inhibition of PI3K activity with LY294002 (an inhibitor of PI3K) resulted in reduced to complete loss of AKT dependent GSK3, phosphorylation (Figure 1B, lanes 2, 4, 6, and 8), further confirming increased activation of the PI3K-AKT pathway in T-LGL that appears to disturb the normal balance of apoptotic and survival signaling important for maintaining cellular homeostasis.
The PI3K activity in T-LGL is dependent on an upstream Src family kinase
Generally, PI3K is induced downstream of activated growth factor or cytokine receptors. Therefore, in the next set of experiments we sought to identify an upstream pathway that may be involved in PI3K activation in T-LGL. Given previous reports of constitutive signal transducer and activator of transcription 3 (STAT3) tyrosine phosphorylation27 and cytokine production3 by T-LGL, we examined the potential role for Src-family kinases (SFKs) in mediating the PI3K activation observed in T-LGL (Figure 2). In particular, Lck and Fyn constitute interesting candidate SFKs based on their relationship with multiple receptor systems and association with lipid rafts in the plasma membrane.28-32 Additionally, raft association places Lck in the proper spatial context to influence PI3K activity.33
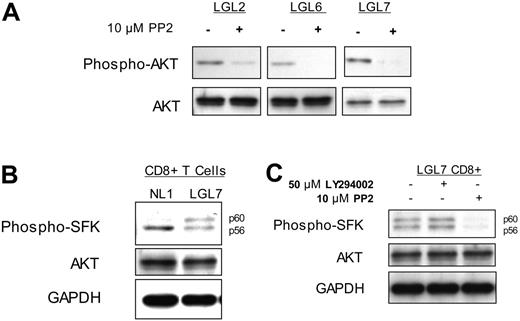
Brief (15-minute) exposure of T-LGL to 10 μM PP2 (an inhibitor of SFKs) resulted in nearly complete loss of phosporylated AKT, while total levels of AKT were not affected (Figure 2A). T-LGL CD8 T cells were purified and lysates analyzed via SDS-PAGE and immunoblotting for SFK activation, as noted by the immunoreactivity with an antibody that detects a conserved phosphotyrosine residue in the catalytic kinase domain (Figure 2B-C). When the Src-family kinase Lck is fully active, its migration on polyacrylamide gels is retarded from an apparent molecular weight of 56 kDa to 60 kDa and provides additional evidence for its activation.34 This doublet of an active SFK was also evident in unstimulated T-LGL CD8 T cells, but absent in control CD8 T cells from healthy donors (Figure 2B), suggesting active SFK signaling in T-LGL.
Subsequently, we hypothesized that the active PI3K acts downstream of the SFK, and inhibition of the former would not affect the latter. Therefore, T-LGL were incubated (15 minutes) without inhibitors (untreated), with 50 μM LY294002, or with 10 μM PP2 prior to lysis and separation on SDS-PAGE for immunoblotting. Although the SFK inhibitor PP2 appeared capable of inhibiting PI3K activity, as shown, the PI3K inhibitor LY294002 failed to inhibit SFK activity in T-LGL (Figure 2C). This observation suggests that in T-LGL, a SFK is activated, eventually leading to downstream activation of PI3K.
The ERK 1/2 MAPK pathway has a high basal level of activation in T-LGL that is PI3K dependent
Constitutive extracellular signal–regulated kinase (ERK) activation has been found in chronic NK lymphoproliferative disease of large granular lymphocytes (NK-LGL), a disorder closely related to T-LGL.35 The probable cause for ERK activation in NK-LGL was ascribed to constitutively active Ras positioned upstream of ERK.
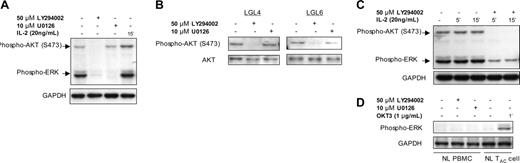
In the T-LGL, we noted an increase in ERK phosphorylation (Figure 3). Since we had shown that PI3K is active in T-LGL, and previously reported that PI3K can regulate ERK activation in T cells,36 the potential relationship between elevated levels of phosphorylated PI3K and ERK was examined with the use of inhibitors that target the respective pathways. T-LGL were incubated with 50 μM LY294002, or 10 μM U0126 (an inhibitor of mitogen-activated protein kinase [MAPK] kinase [MEK], the kinase that phosphorylates and activates ERK) prior to lysis and the lysates were subjected to SDS-PAGE, and immunoblotting. In control T-LGL (not exposed to inhibitors), both AKT and ERK exhibited increased phosphorylation (Figure 3A, lane 1). Upon addition of the PI3K inhibitor, LY294002, there was nearly complete loss of AKT and ERK phosphorylation, whereas with the ERK inhibitor U0126 there is no discernable loss of phospho-AKT reactivity while phospho-ERK is no longer detectable (Figure 3A, lanes 2 and 3, respectively). In the final lane of Figure 3A, lysates derived from T-LGL after exposure to 20 ng/mL IL-2 (a well-characterized and potent inducer of PI3K activity) demonstrated an increased AKT phosphorylation above background levels as well as a higher phospho-ERK signal. Furthermore, even in the presence of IL-2, AKT phosphorylation proved highly sensitive to the inhibitory effects of LY294002 (Figure 3B). When IL-2–stimulated T-LGL were treated with 50 μM LY294002, there was a complete loss of phospho-AKT and marked reduction in phospho-ERK (Figure 3B, lanes 4 and 5).
PI3K activity in T-LGL is dependent on the activation of a Src family kinase. (A) PBMCs from 3 patients with T-LGL were incubated at 37°C for 15 minutes in the presence or absence of the SFK inhibitor PP2 (10 μM), followed by immediate addition of 2 × SDS sample buffer. Cell equivalents (3 × 105)/lane were run on a 10% SDS-PAGE gel, transferred to nitrocellulose, and immunoblotted for phosphorylated AKT (S473) and total AKT. (B) CD8+ T cells isolated from a healthy donor and a patient with T-LGL via RosetteSep were resuspended in RPMI at 5 × 107 cells/mL and incubated at 37°C for 15 minutes, followed by immediate addition of 2 × SDS sample buffer and immunoblotted with an antibody specific for active SFKs. (C) CD8+ T cells isolated from a patient with T-LGL via RosetteSep were resuspended in RPMI at 5 × 107 cells/mL and incubated at 37°C for 15 minutes in the presence or absence of LY294002 (50 μM) or PP2 (10 μM), as indicated. Lysates prepared as for panel B were immunoblotted for phospho-SFK. Total AKT and GAPDH protein levels are shown as loading controls.
PI3K activity in T-LGL is dependent on the activation of a Src family kinase. (A) PBMCs from 3 patients with T-LGL were incubated at 37°C for 15 minutes in the presence or absence of the SFK inhibitor PP2 (10 μM), followed by immediate addition of 2 × SDS sample buffer. Cell equivalents (3 × 105)/lane were run on a 10% SDS-PAGE gel, transferred to nitrocellulose, and immunoblotted for phosphorylated AKT (S473) and total AKT. (B) CD8+ T cells isolated from a healthy donor and a patient with T-LGL via RosetteSep were resuspended in RPMI at 5 × 107 cells/mL and incubated at 37°C for 15 minutes, followed by immediate addition of 2 × SDS sample buffer and immunoblotted with an antibody specific for active SFKs. (C) CD8+ T cells isolated from a patient with T-LGL via RosetteSep were resuspended in RPMI at 5 × 107 cells/mL and incubated at 37°C for 15 minutes in the presence or absence of LY294002 (50 μM) or PP2 (10 μM), as indicated. Lysates prepared as for panel B were immunoblotted for phospho-SFK. Total AKT and GAPDH protein levels are shown as loading controls.
ERK signaling in T-LGL is dependent on PI3K activity. (A) T-LGL PBMCs (5 × 107 cells/mL) were untreated (control) or stimulated with IL-2 (20 ng/mL), LY294002 (50 μM), or U0126 (10 μM) for 15 minutes at 37°C. Lysates were prepared by immediate addition of 2 × SDS buffer. After 10% SDS-PAGE and transfer, nitrocellulose membranes were sequentially analyzed for phospho-AKT (S473) and phospho-ERK. (B) T-LGL PBMCs (5 × 107 cells/mL) were untreated or stimulated with IL-2 (20 ng/mL) in the presence or absence of LY294002 (50 μM) for the indicated times at 37°C. Lysates were prepared by immediate addition of 2 × SDS buffer. After 10% SDS-PAGE and transfer, nitrocellulose membranes were sequentially analyzed for phospho-AKT (S473) and phospho-ERK. (C) T-LGL PBMCs (5 × 107 cells/mL) were untreated or with LY294002 (50 μM) or U0126 (10 μM) for 15 minutes at 37°C. Lysates were prepared by immediate addition of 2 × SDS buffer. Samples were subjected to SDS-PAGE and Western blotting for phospho-AKT (S473). For all immunoblots, GAPDH or total AKT were probed to indicate consistency of protein loading between lanes on a given gel. (D) Normal PBMCs and IL-2–activated T cells (TAC) were incubated with LY294002 (50 μM) or U0126 (10 μM) for 15 minutes at 37°C, or with 1 μg/mL OKT3 for 1 minute prior to lysis in 2 × SDS sample buffer. Proteins were separated on an SDS-PAGE gel as in the previous panels, transferred to nitrocellulose, and immunoblotted for phosphorylated ERK.
ERK signaling in T-LGL is dependent on PI3K activity. (A) T-LGL PBMCs (5 × 107 cells/mL) were untreated (control) or stimulated with IL-2 (20 ng/mL), LY294002 (50 μM), or U0126 (10 μM) for 15 minutes at 37°C. Lysates were prepared by immediate addition of 2 × SDS buffer. After 10% SDS-PAGE and transfer, nitrocellulose membranes were sequentially analyzed for phospho-AKT (S473) and phospho-ERK. (B) T-LGL PBMCs (5 × 107 cells/mL) were untreated or stimulated with IL-2 (20 ng/mL) in the presence or absence of LY294002 (50 μM) for the indicated times at 37°C. Lysates were prepared by immediate addition of 2 × SDS buffer. After 10% SDS-PAGE and transfer, nitrocellulose membranes were sequentially analyzed for phospho-AKT (S473) and phospho-ERK. (C) T-LGL PBMCs (5 × 107 cells/mL) were untreated or with LY294002 (50 μM) or U0126 (10 μM) for 15 minutes at 37°C. Lysates were prepared by immediate addition of 2 × SDS buffer. Samples were subjected to SDS-PAGE and Western blotting for phospho-AKT (S473). For all immunoblots, GAPDH or total AKT were probed to indicate consistency of protein loading between lanes on a given gel. (D) Normal PBMCs and IL-2–activated T cells (TAC) were incubated with LY294002 (50 μM) or U0126 (10 μM) for 15 minutes at 37°C, or with 1 μg/mL OKT3 for 1 minute prior to lysis in 2 × SDS sample buffer. Proteins were separated on an SDS-PAGE gel as in the previous panels, transferred to nitrocellulose, and immunoblotted for phosphorylated ERK.
While the PI3K-AKT pathway has been reported to be dependent on ERK,37 this relationship is likely influenced by the cellular context. In T-LGL, as in other T cells,36 the hierarchic nature of this association appeared to be completely ERK dependent on PI3K (Figure 3C). In the 2 additional patients with T-LGL shown here, we further substantiated the findings that the ERK inhibitor U0126 failed to impede the PI3K pathway (Figure 3C, lanes 3 and 6). Importantly, the rapid decline in phosphorylated AKT within 15 minutes of LY294002 addition suggests that the increased levels of phospho-AKT noted in T-LGL are not due to a gross defect in the phosphatase activity that normally counteracts PI3K pathway activation (Figure 3C, lanes 2 and 4), as in the T leukemia cell line Jurkat.38 Furthermore, in each case of T-LGL that we have studied, elevated levels of PI3K-dependent phosphorylated ERK have been observed.
Importantly, we do not observe phosphorylated ERK in unstimulated T cells from healthy donors (Figure 3D), as we have previously shown.36 After culturing with IL-2, these T cells are capable of inducing phospho-ERK upon T-cell receptor (TCR) stimulation via OKT3, as shown in the last lane of Figure 3D.
Inhibition of PI3K in T-LGL increases the spontaneous rate of apoptosis
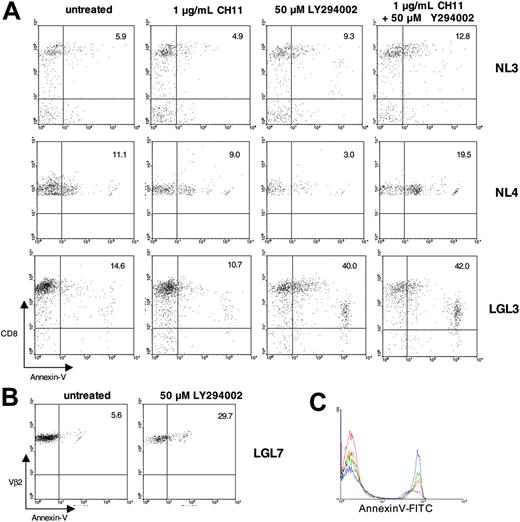
In view of the fact that PI3K has a central role in regulating cellular metabolism and apoptosis, it is possible that abnormally increased activation of the PI3K pathway is involved in the accumulation of T-LGL cells and their relative resistance to apoptosis. For example, T-LGL express Fas receptor, yet fail to undergo anti-Fas or FasL-mediated apoptosis.2 We hypothesized that since PI3K was shown to impede Fas signaling,39 inhibition with LY294002 would lead to increased Fas sensitivity in T-LGL and enhance anti-Fas induced apoptosis (Figure 4). PBMCs from healthy donors and T-LGL patients were cultured with 1 μg/mL CH11 (anti-Fas IgM), 50 μM LY294002, or a combination of 1 μg/mL CH11 + 50 μM LY294002 overnight. After 20 hours, cells were surface-stained for CD8 and annexin V and analyzed via flow cytometry. In PBMCs from both healthy donors and patients with T-LGL, anti-Fas treatment alone did not result in significant loss of viability or increased annexin V staining (Figure 4A). When normal PBMCs were treated with 50 μM LY294002 there was no appreciable increase in annexin V–positive CD8 T cells, but a modest increase in annexin V–positive CD8 T cells was observed when LY294002 was combined with anti-Fas (Figure 4A, top and middle rows). T-LGL showed a strikingly contrasting behavior; when PBMCs from patients with T-LGL were treated with 50 μM LY294002 alone there was a considerable increase in annexin V–positive CD8 T cells, and there was only a slight increase in annexin V staining when LY294002 and anti-Fas were combined (Figure 4A, bottom row). These data suggested that the increased PI3K activity in T-LGL is essential for survival, and the mechanism is likely distinct from simply impeding Fas-mediated apoptosis. In addition, when the ERK pathway was inhibited with U0126, there was only a very mild increase in annexin V staining (data not shown), suggesting that additional PI3K-mediated pathways are responsible for this prosurvival phenotype in T-LGL.
Apoptosis induction after PI3K pathway inhibition in T-LGL. (A) Healthy-donor PBMCs and T-LGL PBMCs were incubated overnight in RPMI with 10% FCS at 37°C either untreated or treated with CH11 (anti-Fas; 1 μg/mL), LY294002 (50 μM), or CH11 (1 μg/mL) + LY294002 (50 μM), as indicated. After 20 hours the cells were stained for FITC–annexin V and PC5-CD8 and analyzed via FACS. For analysis, cells were gated on the lymphocyte population based on forward and scatter profile. The percentage of CD8+ cells that are annexin V positive are indicated by the numbers in the top right quadrants. (B) PBMCs from a T-LGL patient with a large Vβ2 clonal expansion (90% of all PBMCs) were incubated overnight in RPMI with 10% FCS at 37°C either untreated, or with 50 μM LY294002. After 20 hours, the cells were stained for FITC–annexin V and PE-Vβ2 and analyzed via FACS. For analysis, cells were gated on the Vβ2 population. The percentage of Vβ2+ cells that are annexin V positive are indicated by the numbers in the top right quadrants. (C) T-LGL PBMCs were incubated overnight in RPMI with 10% FCS at 37°C either untreated (red) or with 6.25 μM LY294002 (gray), 12.5 μM LY294002 (orange), 25 μM LY294002 (green), or 50 μM LY294002 (blue). After 16 hours the cells were stained for FITC–annexin V and PC5-CD8 and analyzed via FACS. For analysis, cells were gated on the lymphocyte population based on forward and scatter profile.
Apoptosis induction after PI3K pathway inhibition in T-LGL. (A) Healthy-donor PBMCs and T-LGL PBMCs were incubated overnight in RPMI with 10% FCS at 37°C either untreated or treated with CH11 (anti-Fas; 1 μg/mL), LY294002 (50 μM), or CH11 (1 μg/mL) + LY294002 (50 μM), as indicated. After 20 hours the cells were stained for FITC–annexin V and PC5-CD8 and analyzed via FACS. For analysis, cells were gated on the lymphocyte population based on forward and scatter profile. The percentage of CD8+ cells that are annexin V positive are indicated by the numbers in the top right quadrants. (B) PBMCs from a T-LGL patient with a large Vβ2 clonal expansion (90% of all PBMCs) were incubated overnight in RPMI with 10% FCS at 37°C either untreated, or with 50 μM LY294002. After 20 hours, the cells were stained for FITC–annexin V and PE-Vβ2 and analyzed via FACS. For analysis, cells were gated on the Vβ2 population. The percentage of Vβ2+ cells that are annexin V positive are indicated by the numbers in the top right quadrants. (C) T-LGL PBMCs were incubated overnight in RPMI with 10% FCS at 37°C either untreated (red) or with 6.25 μM LY294002 (gray), 12.5 μM LY294002 (orange), 25 μM LY294002 (green), or 50 μM LY294002 (blue). After 16 hours the cells were stained for FITC–annexin V and PC5-CD8 and analyzed via FACS. For analysis, cells were gated on the lymphocyte population based on forward and scatter profile.
One T-LGL patient in particular (LGL7) had a large clonal CD8 T-cell expansion with 90% of all PBMCs composed of TCR-Vβ2–restricted CD8 T cells. Similar to the experiments in Figure 4A, PBMCs from this patient were incubated with 50 μM LY294002 for 20 hours. Upon flow cytometric (FACS) analysis of Vβ2 T cells, approximately 6% of the cells in the untreated group were annexin V positive, whereas 30% of the LY294002-treated cells were rendered apoptotic as assessed by the annexin V staining (Figure 4B). For experiments involving inhibition of PI3K, we used LY294002 at a concentration of 50 μM, previously shown to be optimal for inhibiting the PI3K-AKT pathway, including AKT phosphorylation at S473.40-44 However, to confirm this in our experimental setting, we treated T-LGL cells with increasing concentrations of LY294002 and show increasing annexin V–positive staining by flow cytometry, as indicated in the figure legend (Figure 4C).
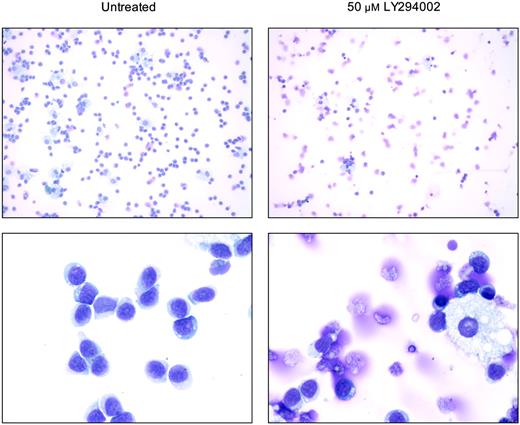
In addition to the analysis of apoptotic propensity of T-LGL cells using annexin V flow cytometry, morphologic changes that occur upon treatment with the PI3K inhibitor LY294002 were analyzed on cytospin preparations (Figure 5). As demonstrated in the control T-LGL PBMCs without inhibitors, cultures contained mostly lymphocytes with only occassional macrophages (Figure 5, left panels). When such cultures were treated with LY294002, there was a dramatic change in the morphology of lymphocytes, with increased appearances of apoptotic cells and fragments of cells (Figure 5, right panels). Simultaneously, residual macrophages in the LY294002-treated samples appeared unchanged, suggesting a selective susceptibility of T-LGL to PI3K inhibition. When PBMCs from healthy donors were analyzed under the same conditions, the extensive loss of lymphocyte viability seen with T-LGL was absent (data not shown).
Discussion
Aggressive chemotherapy directed at eradicating the clonal CTL in T-LGL has not been effective in eradicating the malignant clone.45,46 In contrast, use of immunosuppressive regimens such as low-dose methotrexate, oral cytoxan, and cyclosporine have shown greater utility.47-50 Our results demonstrating that signal transduction pathways initiated by PI3K are active and critically important for cell survival in T-LGL provide a novel set of targets that could greatly complement current treatment strategies.
A key issue regarding T-LGL pathogenesis is the question of what mechanisms are supporting the ability of the pathologic CTLs to avert normal homeostatic apoptosis. Based on previous reports of constitutive cytokine production by T-LGL, we proposed the mechanism attenuating homeostatic apoptosis might involve signaling through a cytokine receptor and activity of an antiapoptotic signal transduction pathway. In agreement with such a hypothesis, the proinflammatory cytokines produced in T-LGL are all capable of activating the PI3K pathway, a key mediator of cellular survival (reviewed in Vivanco and Sawyers15 ). In this respect, our results demonstrate that PI3K is active in T-LGL, and inhibition of this pathway sensitizes the CTLs to apoptosis. However, it is important to note that LY294002 has recently been shown to inhibit enzymes related to PI3K at similar concentrations; thus, we must acknowledge alternative pathways that may be contributing to induction of apoptosis in T-LGL treated with LY294002. Careful consideration of these other enzymes inhibited by LY294002 helps to clarify this issue. Mammalian target of rapamycin (mTOR), a downstream target of the PI3K pathway, is not likely playing a major role because treatment of T-LGL cells with rapamycin did not increase annexin V staining (data not shown). DNA-dependent protein kinase (DNA-PK), ataxia telangiectasia mutated (ATM), and ATM-Rad3–related protein (ATR) are also PI3K-related kinases susceptible to LY294002 inhibition, but these proteins are not likely to be involved in the apoptosis we observe, given their known mechanisms of action. The most relevant alternatives to the PI3K-AKT pathway in our study are members of the Pim kinase family, also susceptible to LY294002 inhibition. However, we were unable to detect Pim kinase in any CTL lysates from our patients with T-LGL (data not shown). Thus, taking into account the increased activity of the PI3K pathway that we have shown, and its well-characterized inhibition by LY294002, we favor this pathway as a key mediator of T-LGL resistance to apoptosis.
PI3K inhibition induces morphologic changes in T-LGL consistent with apoptosis. Untreated (control) T-LGL are noted in the left panels intermixed with occasional macrophages. The right panels show images of Wright-stained T-LGL PBMCs that had been treated for 20 hours with LY294002 (50 μM). Apoptotic cells are seen in the LY-treated T-LGL. Original magnifications: low power, × 200; high power, × 1000 (oil).
PI3K inhibition induces morphologic changes in T-LGL consistent with apoptosis. Untreated (control) T-LGL are noted in the left panels intermixed with occasional macrophages. The right panels show images of Wright-stained T-LGL PBMCs that had been treated for 20 hours with LY294002 (50 μM). Apoptotic cells are seen in the LY-treated T-LGL. Original magnifications: low power, × 200; high power, × 1000 (oil).
Membrane receptor systems which use recruited tyrosine kinase activity, such as cytokine receptors, primarily activate the class IA family of PI3K consisting of a 110-kDa catalytic subunit and an 85-kDa regulatory subunit.51 Upon receptor phosphorylation, the p85 subunit is recruited to the receptor complex via src homology 2 (SH2) domain interactions and places the p110 subunit in the proper spatial context to phosphorylate the D-3 position of phosphatidylinositol in the plasma membrane. This new lipid moiety acts as a focal point on the inner leaflet of the plasma membrane for initiating signal pathways, such as the serine/threonine kinase AKT (also known as protein kinase B). AKT has many targets, including apoptosis signaling kinase-1,52 MDM-2,53 TSC2,54,55 GSK-3,26 BAD,56,57 AFX,58,59 and FKHR.60-64 Thus, the PI3K pathway acting in part through AKT controls cell survival, proliferation, and metabolism on many levels and is an obvious point of interest for a disease involving dysregulated apoptosis, such as T-LGL.
Our observation that a SFK is active and necessary for PI3K activity in T-LGL suggests there is an upstream receptor that is activated and initiates this kinase activity. Since cytokine receptors do not display intrinsic kinase activity, they rely on associated Janus kinase (JAK) and interactions with Src family kinases. Previously, inhibition of JAK2/JAK3 via the tyrphostin AG-490 was shown to increase apoptosis, and this was attributed to the loss and Mcl-1 induction by STAT3.27 We have confirmed the findings of Epling-Burnette et al27 in detecting phosphorylated STAT3 (Y705) in T-LGL. Under the same conditions that PP2 inhibited SFK phosphorylation, and LY294002 inhibited AKT phosphorylation, neither drug inhibited phosphorylation of STAT3 (data not shown). However, the normal kinetics regulating STAT3 dephosphorylation has not been established in these cells, and the early time-point investigated for the membrane proximal signaling proteins (15-minute incubation with PP2 or LY294002) may not be long enough for phosphorylated STAT3 to efficiently interact with the relevant tyrosine phosphatase. We are currently investigating potential pathway crosstalk between JAK-STAT and PI3K-AKT signaling.
In addition to JAKs, SFKs (including Lck in T cells) have been shown to activate STAT proteins.65,66 A further interest in SFKs relates to their ability to influence the activity of the PI3K pathway by inhibiting PTEN, the lipid phosphatase that dephosphorylates phosphatidylinositol-3-phosphate and attenuates PI3K signaling.67 Thus, our finding of SFK-dependent PI3K activity strengthens the hypothesis that T-LGL is a disease of dysregulated homeostatic apoptosis.
Upon further analysis of PI3K-dependent signaling in T-LGL, we discovered that the MAPK ERK is phosphorylated in the clonal CTLs from patients with T-LGL. As one of the most prominent effectors of the PI3K pathway, AKT has been shown to regulate Raf-1, an upstream kinase in the ERK pathway, via an intermediate kinase, p21-activated kinase (PAK).68-70 Since we have previously shown that the PI3K-AKT pathway regulates the threshold for ERK activation in primary T cells, and in light of the recently described role for the Ras/Raf-1/ERK pathway in NK-LGL, we were interested to test the hypothesis that ERK inhibition in T-LGL would increase susceptibility to apoptosis. However, unlike the reported finding in NK-LGL, our experiments indicate that inhibition of ERK in T-LGL resulted in only a very mild increase in apoptosis. Therefore, we contend that PI3K-mediated activation of ERK in T-LGL is not directly related to the ability of the clonal CTLs to avert apoptosis. The differential role of ERK activation in T-LGL versus NK-LGL may present an opportunity to further explore the pathophysiology of these related lymphoproliferative disorders and warrants further study.
In conclusion, we find that elevated PI3K activity in T-LGL likely plays an important role in the ability of the pathologic cells to avoid homeostatic apoptosis, since inhibition of this pathway leads to apoptosis in the population of cells harboring the pathologic clone. More importantly, the activity of this pathway may represent a kind of “Achilles heel” for T-LGL in that PI3K inhibitors alone are quite effective at inducing spontaneous apoptosis in the clonal CTLs after a short incubation. As novel inhibitors of PI3K and its downstream components become available for clinical applications, it will be interesting to further investigate their efficacy in T-LGL.
Prepublished online as Blood First Edition Paper, February 16, 2006; DOI 10.1182/blood-2005-08-3076.
Supported in part by grants RO1 HL073429-01 and RO1 CA113972-01 awarded to J.P.M. A.E.S. is supported by the Medical Scientist Program (NIH 5 T32 GM00 7250-29), Case Western Reserve University School of Medicine, Cleveland, OH.
A.E.S. designed research, performed research, analyzed data, and wrote the paper; J.J.P. performed research and analyzed data; M.W.W. performed research and analyzed data; and J.P.M. designed research, provided study material/patients, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Tom Loughran Jr for reviewing this manuscript, Dr P. K. Epling-Burnette for insightful discussions, and members of the Maciejewski lab for technical support.