Abstract
Chemokines are key controllers of cell trafficking and are involved in numerous pathologic and inflammatory conditions. However, the fate of a chemokine ligand, once it is endocytosed with its receptor, remains obscure. Here, using chemokine–tumor antigen fusion constructs, we demonstrate for the first time that chemokines are internalized to early/late endosomal and lysosomal compartments through a clathrin-dependent process and subsequently delivered to the cytosol for proteasomal processing, facilitating efficient cross-presentation to the TAP-1–dependent MHC class I processing pathway. These data not only elucidate the intracellular fate of chemokine ligands upon receptor uptake, but also demonstrate the superior carrier potency of chemokines for delivering self-antigens to both class I and II processing pathways to induce CD8+ and CD4+ T-cell responses.
Introduction
Heterotrimeric G-protein–coupled 7-transmembrane–domain chemokine receptors (GPCRs) play a pivotal role in both homeostatic cell trafficking and a wide variety of inflammatory processes.1,2 Immature dendritic cells (iDCs) are recruited to the periphery or to sites of inflammation and infection through preferentially expressed chemokine receptors, including CCR1, CCR2, CCR5, and CCR6.3,4 Chemokines upon receptor ligation induce phosphorylation and endocytosis of chemokine receptors through clathrin-coated vesicles using β-arrestin adaptors,5,6 although viral chemokine receptors, such as US28, are endocytosed independently of β-arrestins.7 The internalized receptors are subsequently dephosphorylated and recycled back to the cell surface or targeted for degradation.5,6,8 CCR5 has been transported to early endosomes and subsequently recycled to the cell surface bypassing the Golgi apparatus and late endosomes, and this process does not involve protein synthesis.6 Moreover, the fate of the internalized receptor and the bound ligand may actually be regulated by the strength of the ligand-induced signaling or the nature of the ligand itself. For example, CCR5 is endocytosed through clathrin-coated vesicles upon binding to RANTES or AOP-RANTES, and although the latter drives CCR5 to a degradation pathway, RANTES-bound CCR5 is recycled to the cell surface.6 While internalized receptors have been shown to be degraded by proteasomes, which are considered major regulators of cytokine receptor expression,9,10 little is know about what happens to the internalized chemokine ligand during this process.
We have recently demonstrated that effective adaptive immunity against poorly immunogenic self-tumor antigens can be enhanced by targeting their uptake by antigen-presenting cells (APCs) through their cell-surface chemokine receptors. Mice immunized with chemoattractants fused with nonimmunogenic lymphoma antigens elicited potent protective and therapeutic antitumor responses against challenge with a lethal dose of syngeneic tumor cells.11,12 These vaccines do not require the use of any additional adjuvants as potent immune responses are elicited following injections of recombinant proteins alone or DNA constructs encoding fusion proteins. However, it is essential that the tumor antigens are physically fused with a functionally active chemokine, as immunization of mice with free nonfused chemokine plus antigen or with fusion constructs lacking the ability to bind chemokine receptors fails to elicit tumor-specific immune responses in vivo.11 These data suggest that vaccine antigens are taken up, processed, and presented by APCs after binding and internalization through chemokine receptors. In support of this hypothesis, we have recently demonstrated that chemokine receptors do indeed facilitate the uptake and processing of tumor antigens to induce efficient CD4+ T-cell responses both in vitro and in vivo using the MHC class II antigen processing pathway.13 Through the use of inhibitors of intracellular trafficking, chemoattractant fusion proteins, but not antigen alone, were found to be processed and presented through early/late endosomal and Golgi compartments. How such fusion protein processing pathways may intersect with the established antigen processing pathways remains unclear.
Based on the observation that proteasomes play an essential role in antigen processing and presentation through MHC class I molecules14 in the internalization and down-regulation of several cell-surface cytokine receptors,9,10 we have hypothesized that, upon internalization, chemokine fusion proteins may also be processed and/or degraded using similar machinery as the MHC class I processing pathways. The possibility has been indirectly supported by our recent in vivo data showing that DNA vaccines encoding chemoattractant fusions elicited systemic and mucosal CD8+ cytotoxic T lymphocyte (CTL) responses to the HIV-1 Env protein.15 In the present study, we provide direct evidence that chemokine receptors do indeed deliver antigens to the MHC class I processing pathway and facilitate TAP-1–dependent antigen cross-presentation to CD8+ T cells. These data not only demonstrate the usefulness of chemokines in cancer vaccine development,11,12 which requires induction of effective CD4+ and CD8+ T-cell responses against nonimmunogenic or weakly immunogenic self-tumor antigens, but also provides new insight into the biology of chemokine and chemokine receptor interactions.
Materials and methods
Fusion-gene cloning and plasmid construction
Cloning strategy for murine MIP3α/CCL20, murine β-defensin 2 (mDF2β), and MC148 was previously described.11,12 The truncated human gp100 DNA fragment (a generous gift from Dr Paul Robins, NCI/NIH; GenBank no. P40967) corresponding to residues from 22 to 236 aa's was cloned using primers PRhPmel17-1, ATACATATGCTCGAGGCTAGAAAAGTACCCAGAAACCA, and PRhPmel17-R1, AAAAGATCTCTCTAGATTTCTCAGGAAGTGCTTGT. Murine OFA-iLRP (GenBank no. AF140348) was cloned from murine A20 B-cell lymphoma (American Type Culture Collection [ATCC], Manassas, VA) using the primers PRmOFA-1, CATACCATGGTCGACGGAGCCCTTGACGTCCTGCAG, and PRmOFA-R1, TTAGGATCCGGACCACTCAGTGGTGGCT. All constructs were verified by the DNA sequencing (Fidelity Systems, Gaithersburg, MD).
Recombinant fusion proteins and peptides
Fusion proteins were purified from inclusion bodies as described previously.11,16 The integrity and purity (> 90%) of recombinant proteins were tested by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and Western blot hybridization with 9E10 anti–c-myc mAb (Sigma, St Louis, MO).
The peptides human gp10025-33 (KVPRNQDWL), mouse gp10025-33 (EGSRNQDWL),17 iLR58-66 (LLLAARAIV), iLR60-68 (LAARAIVAI),18 and A20 106-114 (DYWGQGTEL, a control peptide for H-2Kd)19 were all synthesized by Peptide Technologies (Washington, DC) to a purity more than 99% by high-performance liquid chromatography (HPLC) and amino acid analysis.
Cell lines and immunizations
The A20 B-cell lymphoma (H-2d, OFA-iLRP positive), MOPC315 plasmacytoma (H-2d, OFA-iLRP negative), and EL-4 thymoma (H-2b, gp100 negative and OFA-iLRP positive) cell lines were purchased from ATCC. The B6/129 macrophage cell line (H-2d, CCR6+) was a generous gift from Dr Howard Young (National Cancer Institute, Frederick, MD). The B16 melanoma (H-2b) was obtained from the National Cancer Institute tumor repository and has been shown to express gp100.17 Murine bone marrow (BM)–derived DC preparation has been previously described.20 Immature DCs on days 4 to 5 typically expressed CD11+ (69%), B7.2+ and I-Ab+ (21%), B7.2– and I-Ab+ (18%), and CD40+ (27%); and mature DCs expressed CD11c+ (87%), B7.2+ and I-Ab+ (62%), B7.2– and I-Ab+(3%), and CD40+ (87%).
All animals were bred or housed at the National Institute of Aging animal facility, Baltimore, MD. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals.21 The TCR transgenic pmel-1 (Vα1Vβ13 T-cell receptor for H-2Db–restricted mouse and human gp100 epitope) mice have been described previously.17 C57BL/6 (H-2b), BALB/c (H2d), and B6.129S2-Tap1<tm1Arp>/J (TAP-1 gene knock out) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). For tumor protection study, 6- to 8-week-old female C57BL/6 were immunized with the Helios Gene Gun System (Bio-Rad, Hercules, CA) with 1 to 2 μg plasmid DNA 3 times every 2 weeks as described. Two weeks after the last immunization, mice were challenged subcutaneously with 1 × 105 B16 melanoma cells and tumor size was measured (mm2). Survival between groups was determined using a nonparametric log-rank test (BMDP statistical software, Los Angeles, CA).
Preparation of immune effector cells and in vitro activation of T cells
Mice were vaccinated subcutaneously twice at 3-week intervals with 10 μg human gp10025-33 or iLR58-66 peptides, emulsified in 100 μL incomplete Freund adjuvant (IFA). Three weeks after the second vaccination, splenocytes were cultured with 20 IU/mL rhIL-2 and 1 μg/mL corresponding peptide (mgp10025-33 and iLR58-66, respectively) and used on days 5 to 7 after the initiation of the culture.17
In vivo uptake of fusion proteins
C57BL/6 mice (4 mice/group) were immunized intraperitoneally twice at 3-week intervals using the Helios Gene Gun System (Bio-Rad) with 1 to 2 μg DNA (MIP3α-D-gp100, MIP3α-gp100, and mDF2β-gp100) or with 10 μg hgp10025-33 peptide in 100 μL IFA or 100 μL PBS. Three weeks after the second vaccination, splenocytes were harvested, irradiated (20 gray), and mixed directly (without any additional protein stimulation or incubation) with pmel-1 T cells in 96-well round-bottom plates at an effector-target (E/T) ratio of 5:1 for 24 hours to measure IFN-γ release.
Chemokine receptor binding
The ligand binding-internalization assays were performed with iDCs or splenocytes (1 × 105) blocked with mouse serum in PBS containing 2% BSA (PBSst). Fusion proteins (10-50 μg/mL) were incubated in complete medium for one hour at 37°C or at 4°C. To detect bound proteins, the cells were incubated with anti–c-myc mAb or isotype-matched, purified mouse IgG1, followed with α-mouse Ig-FITC mAb incubation (Jackson ImmunoResearch Laboratory, Bar Harbor, ME) for 20 minutes each, and then fixed with 1% paraformaldehyde. The binding-internalization was assessed via flow cytometry on a FACScan using CellQuest software (both from Becton Dickinson, Franklin Lakes, NJ).
Confocal microscopy
B6/129 cells were cultured in covered glass-bottom dishes (MatTek, Ashland, MA) for 2 days as described elsewhere.22 Briefly, cells were incubated with 25 μg/mL fusion proteins in RPMI-1640 containing 10% FBS for one hour at 37°C or at 4°C; fixed with 3.7% formaldehyde; and permeabilized for 5 minutes with 0.2% Triton X-100. Cells were incubated with mouse α–c-myc mAb (clone 9E10; Sigma), and rabbit anti–LAMP-1 antibody (H-228) or rabbit anti–Clathrin HC (H-300) (both from Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit anti–proteasome 20S subunit alpha-5 (Affinity BioReagents, Golden, CO) for 1 hour at 37°C, and then for 30 minutes at 37°C with secondary antibodies, goat anti–mouse IgG conjugated to Alexa Flour 488, and goat anti–rabbit IgG conjugated to Alexa Flour 568 (Molecular Probes, Eugene, OR). Confocal images were acquired with a 63 × objective on a Zeiss LSM 410 confocal system (Carl Zeiss, Heidelberg, Germany), and were then processed using Adobe Photoshop (Adobe Systems, San Jose, CA).
Intracellular antigen processing
Splenocytes or iDCs from naive C57Bl/6 or BALB/c mice, were incubated overnight with various concentrations of fusion protein (0.01-1 μg/mL). The treated APCs were subsequently irradiated (20 gray), washed twice with PBS to remove unbound proteins, and then cocultured with specific effector cells. Some APCs were treated overnight with chemokine fusions together with various inhibitors: pertussis toxin (PTX, 2.5 ng/mL), sucrose (0.4 M), brefeldin A (500 μM), leupeptin (5 μg/mL), chloroquine (50, 10, and 1 μM), lactacystin (50, 10, and 1 μM), wortmannin (100 and 50 nM), and NH4Cl (40 and 20 nM). All reagents were purchased from Sigma.
Results
Chemoattractant fusion proteins retain their functional activity
We have produced recombinant proteins, which encoded chemokines physically fused with established tumor antigens, including the melanoma-associated antigen, gp100, and tumor-associated embryonic antigen, OFA-iLRP (Figure 1A). The chemokine moieties used in this study have been selected based on the receptors through which they mediate their biologic effects and their selective expression on APCs. For example, murine MIP3α/CCL20 targets CCR6 preferentially expressed on iDCs, while the viral chemokine antagonist MC148 binds to CCR8 expressed on a variety of APCs. Control constructs consisted of either gp100 or OFA antigens alone or fused with an inactive non-CCR8 binding form of MC148.23 In concordance with our previous reports,11 each of these chemokine fusion constructs with the gp100 and OFA antigens retained its chemokine receptor binding and signaling properties despite being linked to a relatively large tumor antigen. MIP3α-gp100 and MIP3α-mOFA all specifically bind to CCR6 expressed on bone marrow–derived immature DCs (BM iDCs; Figure 1B) and on murine splenocytes (SPs; data not shown). Similar to our previous reports,23 the MC148 fusion proteins bind only to murine BM iDCs, not SPs, while the control proteins, namely gp100 or the mutant MC148-D-mOFA, failed to bind any APC population examined (data not shown). While no significant internalization was detected at 4°C, MIP3α-mOFA or MIP3α-gp100 was quickly internalized (Figure 1B-C), and, found intracellularly, foci colocalized with lysosomal marker LAMP-1 (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article) when APCs were incubated at 37°C. These data suggest that the fusion proteins not only retained the functional properties of their nonfused chemokine counterparts, but also were efficiently internalized to various intracellular compartments, including lysosomes, where, as we recently reported, chemokine-fused antigens would be processed and presented to MHC class II molecules.13 On the other hand, exogenous antigens are known to be poorly taken up and presented to MHC class I, a process known as cross-presentation; therefore, it was interesting to test whether chemokine-mediated internalization would also enable antigen cross-presentation.
Fusion-gene cloning and plasmid constructions. (A) Genes for mature sequences of murine MIP3α or the viral chemokine MC148 were fused in-frame with DNA encoding either human gp100 or mouse OFA-iLRP. Control constructs encoded gp100 alone or fused with the mutant nonactive chemokines (MC148-D-hgp100 and MC148-D-mOFA). To enable purification and detection, c-myc and His peptide tags were fused to fusion constructs (Tag). A spacer fragment was inserted between chemoattractant and antigen moieties to enable proper folding of the protein. (B) OFA and gp100 fusion proteins with MIP3α-mOFA and MIP3α-gp100 (50 μg/mL) are bound with CCR6 on the surface of iDCs at 4°C and are induced to internalize at 37°C. Histograms of gated cells (R1 in forward scatter/side scatter plot) are presented. Representative data yielded similar results in 3 independent experiments. (C) Optical z-sections (0.4 μm) were taken of B6/129 macrophages loaded with MIP3α-gp100 at 4°C for 30 minutes and then transferred to 37°C for 0 or 10 minutes before fixation. Cells were stained with either rabbit anticlathrin (H-300) or antiproteasome 20S mAb (red) and mouse antimyc mAb (green) to detect fusion proteins with overlay of both channels showing colocalization in yellow. No significant binding was detected when cells were incubated with mutant chemokine fusion (MC148D-gp100; data not shown). This experiment was repeated 4 times with similar results and a representative experiment is shown.
Fusion-gene cloning and plasmid constructions. (A) Genes for mature sequences of murine MIP3α or the viral chemokine MC148 were fused in-frame with DNA encoding either human gp100 or mouse OFA-iLRP. Control constructs encoded gp100 alone or fused with the mutant nonactive chemokines (MC148-D-hgp100 and MC148-D-mOFA). To enable purification and detection, c-myc and His peptide tags were fused to fusion constructs (Tag). A spacer fragment was inserted between chemoattractant and antigen moieties to enable proper folding of the protein. (B) OFA and gp100 fusion proteins with MIP3α-mOFA and MIP3α-gp100 (50 μg/mL) are bound with CCR6 on the surface of iDCs at 4°C and are induced to internalize at 37°C. Histograms of gated cells (R1 in forward scatter/side scatter plot) are presented. Representative data yielded similar results in 3 independent experiments. (C) Optical z-sections (0.4 μm) were taken of B6/129 macrophages loaded with MIP3α-gp100 at 4°C for 30 minutes and then transferred to 37°C for 0 or 10 minutes before fixation. Cells were stained with either rabbit anticlathrin (H-300) or antiproteasome 20S mAb (red) and mouse antimyc mAb (green) to detect fusion proteins with overlay of both channels showing colocalization in yellow. No significant binding was detected when cells were incubated with mutant chemokine fusion (MC148D-gp100; data not shown). This experiment was repeated 4 times with similar results and a representative experiment is shown.
APCs efficiently process tumor antigens linked to chemokines and facilitate peptide presentation through MHC class I molecules
MIP3α-fused antigens incubated with APCs at nonpermissive temperature (4°C) are found to be bound on the surface of cells often colocalized with clathrins, as demonstrated by FACS analysis (Figure 1B) and confocal microscopy (Figure 1C). In concordance with our reports, this binding required that the antigen was physically linked with the chemokine moiety, since control samples (such as antigen alone or antigen mixed with unlinked free chemokine) or antigen fused with mutant chemokine failed to bind APCs (data not shown). However, within 10 minutes after cells were transferred to permissive temperature (37°C), MIP3α-fused antigens were found in the cytosol and colocalized with proteasomes (10 minutes, Figure 1C), a key requirement for cross-presentation. Thus, this was a first indication that exogenous antigens might use the MHC class I processing pathway if they were presented to the APCs as fusions proteins with chemokines (chemokine-Ag's).
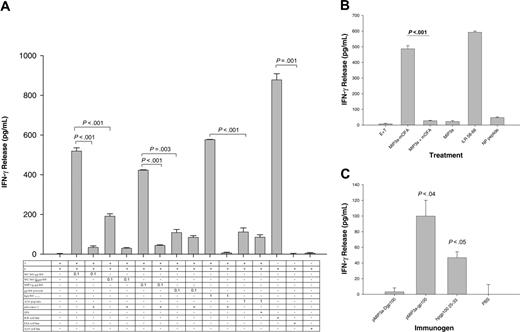
To examine this possibility, APCs derived from naive C57BL/6 mice were incubated overnight with various concentrations of chemokine-fused gp100 (MIP3α-gp100 or MC148-gp100) after which these cells were irradiated and cocultured with immune effector cells (splenocytes) derived from C57BL/6 mice immunized with MHC class I epitope peptide of melanoma antigen gp100 (gp10025-33 peptide). The assumption was that if APCs cross-presented an exogenously added gp100, then they would stimulate MHC class I–specific effector T cells to produce IFN-γ. Indeed, the effector T cells produced significant IFN-γ upon stimulation with syngeneic BM iDCs (Figure 2A) or SPs (Figure S2) pretreated with MIP3α-gp100 or MC148-gp100. In contrast, APCs treated with gp100 protein alone (gp100 protein) or fused with mutant MC148 protein (MC148-D-gp100) failed to induce IFN-γ production (Figure 2A). The specificity of effector cells was confirmed by their reactivity to B16 melanoma cells (H-2b) that naturally express gp100,17 but not to gp100-negative EL-4 thymoma (H-2b) or A20 B-cell lymphoma (H-2d) cells, or by their stimulation with APCs directly pulsed with 1 μg/mL human or mouse gp10025-33 peptide (Figures 2A and S1b).
Next, to exclude that these effects were specific only to gp100 antigen or dependent on mouse strain (C57BL6), similar studies were performed using fusion proteins containing a different tumor antigen, namely OFA-iLRP, expressed by A20 tumor cells and presented to MHC H-2d class I molecules. BALB/c splenocytes were treated with 100 ng/mL MIP3α-mOFA as described above and were subsequently examined for their ability to stimulate effector T cells derived from BALB/c mice immunized with OFA-specific MHC class I peptide (OFA-iLRP58-66) in IFA. As shown in Figure 2B, only APCs pretreated with MIP3α-mOFA induced significant IFN-γ production from the specific T cells, and the response was comparable with one from APCs directly pulsed with OFA-iLRP58-66 peptide. Moreover, the response required that OFA-iLRP was targeted to chemokine receptors via physically linked and functionally active chemokine moiety, since no T-cell stimulation was detected when APCs were pretreated with mutant chemokine fusion (MC148D-mOFA, data not shown) or a mixture of free chemokine and OFA-iLRP (MIP3α+ mOFA, Figure 2B).
Antigen-presenting cells efficiently process tumor antigens linked to chemokines and facilitate peptide presentation through MHC class I molecules. (A) Naive C57BL/6 splenocytes were incubated overnight with 1 μg/mL MIP3α-gp100, MC148-gp100, MC148D-gp100, or gp100 protein alone. The cells were subsequently washed, irradiated, and then cocultured with immune effector splenocytes from C57BL/6 mice (immunized with hgp10025-33/IFA). IFN-γ release was measured after overnight incubation. Effector-cell specificity was validated using splenocytes pulsed with 1 μg/mL hgp10025-33 or an irrelevant A20 peptide, or incubating with B16 melanoma or control A20 lymphoma cells. (B) APCs (splenocytes) from BALB/c mice also cross-present exogenous OFA-iLRP antigen. iDCs were incubated with 0.1 μg/mL MIP3α-mOFA. Control DCs treated with MIP3α fused with an irrelevant tumor antigen, gp100 (MIP3α), alone or mixed with free unlinked MC148-D-mOFA (MIP3α+ OFA) failed to stimulate T cells. The specificity of effector T cells shown by their response to splenocytes directly pulsed with OFA-iLRP–specific MHC class I peptide (iLR58-66), but not control MOPC315 MHC class II peptide (NP peptide). The P value is the comparison between MIP3α-mOFA and MIP3α+ mOFA. Control for effector cells mixed with untreated splenocytes is shown (E + T). (C) Chemokine fusions also presented to MHC class I in vivo. Splenocytes from mice immunized with pMIP3α-gp100 or pmDF2β-gp100; control vaccine pMIP3α-D-gp100; or PBS were irradiated without additional peptide pulsing and mixed with activated effector cells (splenocytes) from pmel-1 mice. Specificity and activity of effector pmel-1 cells have been tested on APCs pulsed with human gp10025-33 peptide (hgp10025-33). A representative experiment is shown from 4 (A-B) and 2 (C) independent experiments, all yielding similar results. Data are averages of triplicate wells, and error bars represent standard deviation of the mean.
Antigen-presenting cells efficiently process tumor antigens linked to chemokines and facilitate peptide presentation through MHC class I molecules. (A) Naive C57BL/6 splenocytes were incubated overnight with 1 μg/mL MIP3α-gp100, MC148-gp100, MC148D-gp100, or gp100 protein alone. The cells were subsequently washed, irradiated, and then cocultured with immune effector splenocytes from C57BL/6 mice (immunized with hgp10025-33/IFA). IFN-γ release was measured after overnight incubation. Effector-cell specificity was validated using splenocytes pulsed with 1 μg/mL hgp10025-33 or an irrelevant A20 peptide, or incubating with B16 melanoma or control A20 lymphoma cells. (B) APCs (splenocytes) from BALB/c mice also cross-present exogenous OFA-iLRP antigen. iDCs were incubated with 0.1 μg/mL MIP3α-mOFA. Control DCs treated with MIP3α fused with an irrelevant tumor antigen, gp100 (MIP3α), alone or mixed with free unlinked MC148-D-mOFA (MIP3α+ OFA) failed to stimulate T cells. The specificity of effector T cells shown by their response to splenocytes directly pulsed with OFA-iLRP–specific MHC class I peptide (iLR58-66), but not control MOPC315 MHC class II peptide (NP peptide). The P value is the comparison between MIP3α-mOFA and MIP3α+ mOFA. Control for effector cells mixed with untreated splenocytes is shown (E + T). (C) Chemokine fusions also presented to MHC class I in vivo. Splenocytes from mice immunized with pMIP3α-gp100 or pmDF2β-gp100; control vaccine pMIP3α-D-gp100; or PBS were irradiated without additional peptide pulsing and mixed with activated effector cells (splenocytes) from pmel-1 mice. Specificity and activity of effector pmel-1 cells have been tested on APCs pulsed with human gp10025-33 peptide (hgp10025-33). A representative experiment is shown from 4 (A-B) and 2 (C) independent experiments, all yielding similar results. Data are averages of triplicate wells, and error bars represent standard deviation of the mean.
The primary responders were MHC class I–dependent CD8+ T cells, since IFN-γ production was significantly reduced in the presence of specific blocking antibodies to MHC H-2b class I (Figure 2A) or anti-CD8 molecules (12.3 ± 1.1 pg/mL IFN-γ), but not control isotype-matched mAb (213.8 ± 4.1 pg/mL IFN-γ). In support, immune effector cells from pmel-1 mice, which contained more than 95% transgenic CD8+ T cells (FACS, data not shown),17 were also stimulated by APCs incubated with MIP3α-gp100 or MC148-gp100, but not with control fusion constructs or gp100 alone (Figure 3). Overall, these data clearly demonstrate that chemokine-fused antigens, targeted to CCR6 and CCR8 receptors expressed on APCs, were processed and cross-presented to MHC class I. This process is quite efficient as APCs incubated with as little as 10 to 100 ng/mL of chemokine-gp100 are capable of stimulating CD8+ T cells in vitro.
Chemokine receptor–targeted antigens are also cross-presented in vivo
Next, we examined if the MHC class I presentation and CD8+ T-cell activation described above also occurred in vivo in mice injected with chemokine-fused vaccine. If this is the case, then APCs derived from immunized mice alone, in the absence of an additional peptide pulsing, should be capable of stimulating the effector CD8+ T cells from pmel-1 mice. Indeed, as described in Figure 2C, freshly isolated splenocytes from mice immunized with constructs expressing MIP3α-gp100 induced significant IFN-γ production from the cocultured pmel-1 effector cells (pMIP3α-gp100, P < .04, Figure 2C). Splenocytes from mock-treated mice or mice immunized with a gp100 fusion construct containing a mutant MIP3α (which cannot bind CCR6) failed to induce any significant IFN-γ production (PBS and pMIP3α-D-gp100, respectively, Figure 2C). Thus, these data demonstrate that APCs are able to take up, process, and present antigens to MHC class I in vivo when their chemokine receptors are targeted.
The intracellular trafficking of chemokine receptors targeted with chemokine fusions is dependent on clathrin-associated vesicles and G-protein signaling. (A). The dependency of clathrin-coated vesicles and G-protein signaling. iDCs were treated overnight with MIP3α-gp100, or MC148-gp100 in presence or absence of 0.4 M sucrose or 2.5 ng/mL pertussis toxin (PTX). Control iDCs were incubated with MC148-D-gp100 and gp100 protein alone. As effector cells, we used splenocytes from pmel-1 mice as described in “Materials and methods.” Specificity of effector cells was tested on iDCs pulsed with hgp10025-33 peptide or control A20 peptide, or mixing with cells such as B16 melanoma, EL4, and A20. Endocytosed chemokine fusion protein is delivered to early/late endosomes and lysosomes. iDCs were treated with 0.1 μg/mL chemokine proteins fused with gp100 (C-D) or OFA-iLRP (shown in μg/mL, B) in the presence or absence of various pharmacologic inhibitors of intracellular organelle trafficking, such as (C) wortmannin (shown in μM) and NH4Cl (nM), or (B,D) leupeptin, chloroquine, or brefeldin A (μM), to test stimulation of activated pmel-1 T cells. (D) Chemokine fusions are cross-presented and stimulate CD8+ T cells. iDCs were treated with 0.1 μg/mL MIP3α-gp100 in the presence or absence of titrated doses of lactacystin (shown in μM), a specific proteasomal inhibitor, to test stimulation of activated pmel-1 T cells. The experiment was repeated 3 to 6 times, and data are average of triplicate wells. Error bars represent the SD of the mean.
The intracellular trafficking of chemokine receptors targeted with chemokine fusions is dependent on clathrin-associated vesicles and G-protein signaling. (A). The dependency of clathrin-coated vesicles and G-protein signaling. iDCs were treated overnight with MIP3α-gp100, or MC148-gp100 in presence or absence of 0.4 M sucrose or 2.5 ng/mL pertussis toxin (PTX). Control iDCs were incubated with MC148-D-gp100 and gp100 protein alone. As effector cells, we used splenocytes from pmel-1 mice as described in “Materials and methods.” Specificity of effector cells was tested on iDCs pulsed with hgp10025-33 peptide or control A20 peptide, or mixing with cells such as B16 melanoma, EL4, and A20. Endocytosed chemokine fusion protein is delivered to early/late endosomes and lysosomes. iDCs were treated with 0.1 μg/mL chemokine proteins fused with gp100 (C-D) or OFA-iLRP (shown in μg/mL, B) in the presence or absence of various pharmacologic inhibitors of intracellular organelle trafficking, such as (C) wortmannin (shown in μM) and NH4Cl (nM), or (B,D) leupeptin, chloroquine, or brefeldin A (μM), to test stimulation of activated pmel-1 T cells. (D) Chemokine fusions are cross-presented and stimulate CD8+ T cells. iDCs were treated with 0.1 μg/mL MIP3α-gp100 in the presence or absence of titrated doses of lactacystin (shown in μM), a specific proteasomal inhibitor, to test stimulation of activated pmel-1 T cells. The experiment was repeated 3 to 6 times, and data are average of triplicate wells. Error bars represent the SD of the mean.
Moreover, immunization of C57BL/6 mice with DNA plasmid constructs expressing either MIP3α-gp100 or mDF2β-gp100, but not mutant MIP3α-D-gp100, also elicit a potent MHC class I–restricted primary T-cell response in vivo, as splenocytes derived from these animals produce significant levels of IFN-γ upon stimulation with APCs pulsed with the mgp10025-33 peptide, but not with an irrelevant peptide (Figure S3). In addition, no responses were elicited in control mice treated with a saline (PBS, Figure S3).
Mechanism of chemoattractant fusion protein cross-presentation: requirement for chemokine receptor endocytosis via clathrin-coated vesicles
Chemokine receptor endocytosis involves G-protein–coupled protein signaling and is mediated through clathrin-coated vesicles using β-arrestin adaptors.5,6 Signaling through many GPCRs can be turned off upon treatment with the Gαi inhibitor, pertussis toxin (PTX),24 while high sucrose hypertonic solution prevents the assembly of clathrin-coated pits and inhibits receptor-mediated endocytosis.25 To assess if chemokine fusion constructs have similar requirements to facilitate antigen uptake and presentation, pmel-1 effector T cells were stimulated with iDCs derived from naive C57BL/6 mice treated with chemokine-gp100 fusion proteins in the presence or absence of PTX or sucrose. While neither PTX nor sucrose affected T-cell stimulation by DCs directly pulsed with human gp10025-33 peptide, T-cell activation in response to MIP3α-gp100– and MC148-gp100–treated iDCs was completely abrogated with PTX and sucrose treatment (Figure 3A). Similarly, the MIP3α-mOFA–induced cross-presentation was completely abrogated by coincubation with PTX (Figure 3B) and sucrose (data not shown). Therefore, these data not only indicate that the effects are antigen and mouse strain independent, but also further support our initial observation that this is an active process that involves a clathrin-dependent chemokine receptor internalization.
Mechanism of cross-presentation of chemokine fusion proteins: requirement for endosomal/lysosomal compartments
Recently we reported that chemokine receptor–mediated MHC class II presentation was drastically suppressed upon coincubation with brefeldin A,13 a fungal metabolite that inhibits vesicle transport of newly synthesized MHC class molecules between the endoplasmic reticulum (ER) and Golgi,26 and monensin, a sodium/potassium/proton ionophore that prevents acidification of intracellular compartments and internalization of CCR5 receptors.27 In the current study, as expected, brefeldin A treatment completely suppressed the CD8+ T-cell activation induced by APCs incubated with MIP3α-mOFA (Figure 3B) or MIP3α-gp100 (Figure 3D). Similarly, T-cell responses were also significantly inhibited when APCs were treated with agents that affected trafficking or antigen processing within endosomal-lysosomal compartments, namely leupeptin and wortmannin (Figure 3B and 3D, respectively). Also, treatment of APCs with NH4Cl, a weak base that caused a generalized increase in the intracellular pH (Figure 3C), or bafilomycin A, a highly selective inhibitor of endosome acidification that blocked endosomal ATPase, (data not shown), or chloroquine, the serine and cysteine protease inhibitor (Figure 3B-D), inhibited T-cell activation in response to MIP3α-OFA or mDF2β-OFA. Therefore, taken together with the results from confocal microscopy analysis (Figure S1), these data suggest that chemokine-mediated cross-presentation required that antigen be first internalized into endosomal and lysosomal compartments.
Mechanism of cross-presentation of chemokine fusion proteins: requirements for the cytosolic degradation and TAP-1 transport
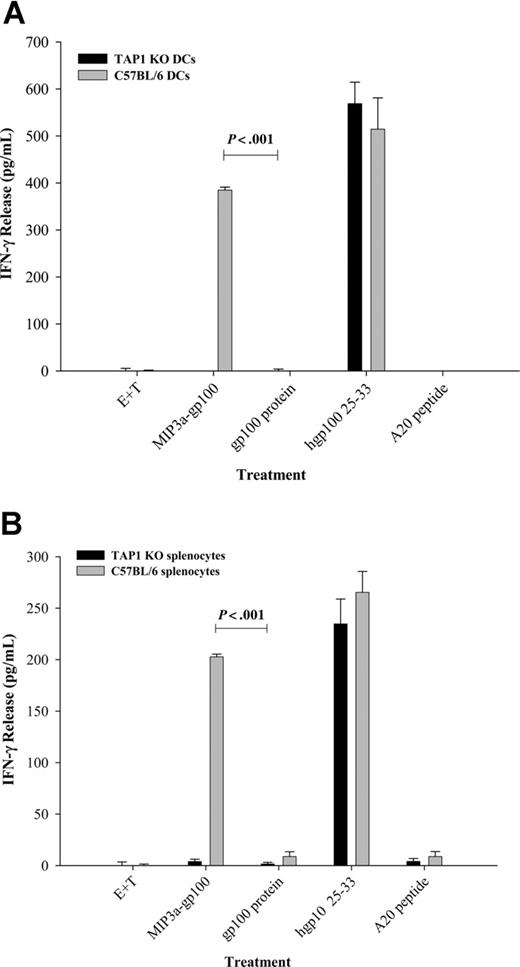
Usually cross-presentation requires that antigen is present in the cytosol and degraded by proteasomes. Therefore, to assess whether chemokine-Ag's used this pathway, we performed a confocal microscopy study that indicated that MIP3α-Ag was found in the cytosol and colocalized with proteasomes (Figure 1C). Moreover, T-cell stimulation by APCs treated with chemokine-Ag's (MIP3α-OFA, Figure 3B; or MIP3α-gp100, Figure 3D) was completely abrogated by coincubation with lactacystin, a specific inhibitor of proteasomal protein degradation. This is not due to toxic effects of lactacystin as lactacystin treatment failed to affect the T-cell stimulatory activity of control APCs pulsed with the processed hgp10025-33 peptide (data not shown) and MHC class II presentation.13 Therefore, these data suggest that chemokine-Ag's are trafficked (from lysosomes, presumably) into the cytosol where they are degraded by proteasomal complexes. The proteasome-degraded peptides are then transported from cytosol to the ER to be associated with MHC class I molecules using TAP-1 machinery. Therefore, to assess involvement of TAP-1, splenocytes and BM iDCs from TAP-1 KO mice were examined for their capacity to cross-present MIP3α-gp100 and stimulate T cells derived from the CD8+ TCR transgenic pmel-1 mice. However, splenocytes or BM iDCs from TAP-1 KO mice incubated with MIP3α-gp100 failed to stimulate T cells (Figure 4A-B black bars), while wild-type APCs facilitated significant IFN-γ expression from pmel-1 effector cells (Figure 4A-B gray bars). It should be noted that this difference is not due to the absence of cell-surface H-2b molecules, as APCs from both mice directly pulsed with hgp10025-33, but not with an irrelevant peptide, stimulated effector cells to produce comparable levels of IFN-γ (Figure 4A-B).
Cross-presentation of chemokine fusion vaccines requires TAP-1 machinery. Immature DCs (A) or splenocytes (B) derived from TAP-1 KO or wild-type C57BL/6 mice were incubated with either MIP3α-gp100 or the gp100 protein alone and tested for their ability to stimulate gp100-specific T cells derived from pmel-1 mice. Control APCs were treated with the active gp100 peptide, hgp10025-33, or irrelevant A20 peptides. IFN-γ release was measured in the supernatants of cells cultured for 24 hours by enzyme-linked immunosorbent assay (ELISA). The data shown are representative of 1 experiment of 3 independent experiments that yielded similar results. Data are average of triplicate wells; error bars represent SD of the mean.
Cross-presentation of chemokine fusion vaccines requires TAP-1 machinery. Immature DCs (A) or splenocytes (B) derived from TAP-1 KO or wild-type C57BL/6 mice were incubated with either MIP3α-gp100 or the gp100 protein alone and tested for their ability to stimulate gp100-specific T cells derived from pmel-1 mice. Control APCs were treated with the active gp100 peptide, hgp10025-33, or irrelevant A20 peptides. IFN-γ release was measured in the supernatants of cells cultured for 24 hours by enzyme-linked immunosorbent assay (ELISA). The data shown are representative of 1 experiment of 3 independent experiments that yielded similar results. Data are average of triplicate wells; error bars represent SD of the mean.
Immunization with chemokine fusion vaccines induces protective antitumor responses
We have previously reported that DNA immunization with constructs expressing chemokine fusions with a nonimmunogenic B-cell tumor antigen elicited a potent therapeutic antitumor immunity against syngeneic B-cell lymphoma challenge.11,12 Given that our chemokine fusion constructs with melanoma antigen, gp100, facilitated efficient antigen cross-presentation in vitro, we next examined if vaccination with DNA constructs encoding chemokine fusion protein can induce a protective response in mice challenged with the very aggressive B16 melanoma, which is known for its resistance to CTLs.28 Ten C57BL/6 mice per group were immunized with gene gun 3 times over a 2-week interval with constructs expressing gp100 fused with active MIP3α (pMIP3α-gp100) or a mutant MIP3α (pMIP3α-D-gp100), or mock immunized with PBS. Two weeks after the last immunization, mice were challenged subcutaneously with a lethal dose of B16 tumor cells and tumor growth was assessed. As shown in Figure 5A, significant inhibition of tumor-cell growth was induced by pMIP3α-gp100 immunizations, while mice immunized with the construct expressing mutant MIP3α fusion (pMIP3α-D-gp100; Figure 5A open circles) failed to suppress tumor growth and improve mouse survival. These data are in agreement with our previous report on DNA vaccine for different tumor antigens indicating that, despite their ability to transduce skin APCs in vivo, antitumor responses depended only on the ability of the vaccine to secrete a fusion protein with a functionally active chemokine moiety.12 We do not think that this response was modulated by “nonspecific” effects of MIP3α since, in agreement with our published data in other tumor models,11,12,23 immunizations with chemokines fused with an irrelevant tumor antigen did not elicit any responses (data not shown). Overall, the medium survival of mice was significantly increased from 17 to 26 days in mice immunized with pMIP3α-gp100 (P = .02) compared with the mutant MIP3α fusion immunized animals.
Discussion
Here we demonstrate that tumor antigens are efficiently delivered to MHC class I processing and presentation pathways (cross-presentation), if linked with chemokines. The process was independent of chemotaxis and required only the receptor internalization, as both agonist chemokine (MIP3α/CCL19) and antagonist ligand (MC148) elicited comparable cross-presentation. In support, we recently reported that mice immunized with DNA constructs expressing tumor antigens fused with either MC148 or proinflammatory host chemokines elicited very comparable protective antitumor responses.23 Given that fusion proteins retain the properties of their respective chemokines, such as the ability to bind the receptor and induce chemotaxis both in vitro and in vivo at doses comparable with native chemokines,11-13 these observations appear to reflect the nature of chemokine receptors rather than changes incorporated when the chemokines are fused with specific antigens. In contrast to a recent report that MDC/CCL22-induced endocytosis of CCR4 in the absence of G-protein coupling,29 chemokine receptor internalization in our hands required Gαi signaling as the process was pertussis toxin sensitive. The data are in agreement with the fact that ligated chemokine receptors are efficiently cleared from the cell surface by endocytosis and are subsequently sorted to lysosomes for degradation, while inactive receptors are recycled back to the cell surface.30
Chemokine fusion vaccination elicits protective antitumor responses in C57BL/6 mice. (A) Ten mice per group were gene-gun immunized 3 times over a 2-week interval with pMIP3α-gp100, pMIP3α-D-gp100, or PBS. Two weeks after the last immunization, mice were challenged subcutaneously with a lethal dose of B16 tumor cells. Tumor growth suppression was subsequently assessed and mice with a tumor larger than 400 mm2 were killed. The data shown are representative of 2 independent experiments that yielded similar results. P = .02. Error bars represent the SD of the mean in 10 mice per group. (B) Mechanism of chemokine-mediated antigen presentation in APCs. Chemokine (MIP3α) is usually internalized upon binding to its chemokine receptor (CCR6) (1-2), and the internalized receptor then can be recycled back to the surface (5). Tumor antigens (TAA), if physically linked with MIP3α (MIP3α-TAA), are preferentially taken up via chemokine receptor–mediated and clathrin-dependent process (1) and internalized into endosomal and lysosomal compartments (2-3), where they can be degraded and presented to MHC class II molecules (3) and subsequently delivered to the cell surface to be presented and activate CD4 T+ cells (4). However, some portion of MIP3α-TAA escapes from (3) to the cytosol (6) where it is degraded by proteasomes (7). The degraded peptides are then transported by TAP-1 to the ER (8) to be loaded to the MHC class I molecules (9) and exposed on the cell surface, thus, inducing CD8+ T cells (11).
Chemokine fusion vaccination elicits protective antitumor responses in C57BL/6 mice. (A) Ten mice per group were gene-gun immunized 3 times over a 2-week interval with pMIP3α-gp100, pMIP3α-D-gp100, or PBS. Two weeks after the last immunization, mice were challenged subcutaneously with a lethal dose of B16 tumor cells. Tumor growth suppression was subsequently assessed and mice with a tumor larger than 400 mm2 were killed. The data shown are representative of 2 independent experiments that yielded similar results. P = .02. Error bars represent the SD of the mean in 10 mice per group. (B) Mechanism of chemokine-mediated antigen presentation in APCs. Chemokine (MIP3α) is usually internalized upon binding to its chemokine receptor (CCR6) (1-2), and the internalized receptor then can be recycled back to the surface (5). Tumor antigens (TAA), if physically linked with MIP3α (MIP3α-TAA), are preferentially taken up via chemokine receptor–mediated and clathrin-dependent process (1) and internalized into endosomal and lysosomal compartments (2-3), where they can be degraded and presented to MHC class II molecules (3) and subsequently delivered to the cell surface to be presented and activate CD4 T+ cells (4). However, some portion of MIP3α-TAA escapes from (3) to the cytosol (6) where it is degraded by proteasomes (7). The degraded peptides are then transported by TAP-1 to the ER (8) to be loaded to the MHC class I molecules (9) and exposed on the cell surface, thus, inducing CD8+ T cells (11).
The fate of the internalized chemokine during receptor internalization remains unknown, although human CCL3 bound to CCR5 has been shown to recycle back to cell surface.8,31,32 Our data demonstrate that the fate of the internalized chemokines (chemokine-Ag's) is as follows: some proportion is degraded in endosomal/lysosomal compartments (for MHC class II presentation),13 while the rest escape to the cytosol where they are degraded by proteasomes to be presented for MHC class I cross-presentation (Figure 5B). However, the MHC class I cross-presentation requires that chemokine-Ag's first enter into the functional active endo/lysosomes, since the response was completely abrogated by use of the specific inhibitors. For example, chemokine receptor internalization and antigen processing in response to treatment with chemokine-Ag's were significantly blocked by the addition of the inhibitors of endosomal acidification, chloroquine and NH4Cl, bafilomycin A1, or wortmannin. A mechanism of escape from the endo/lysosomes is not known. Requirement for lysosomal proteases may suggest that chemokine-Ag's need to be first preprocessed in the endo/lysosomal compartments before being transferred to the cytosol to be further degraded by proteasomes. However, the fact that cells can be killed by cytosolic delivery of RNases linked to chemokines suggests that at least some intact chemokine-Ag's escaped to the cytosol (A.B. and D.B., unpublished data). On the other hand, the intracellular fate of chemokine/chemokine receptor may be determined by the binding strength. For example, CCL3 is more readily dissociated from D6 during vesicle acidification and is subsequently degraded faster when compared with the relatively acid-resistant CCR5/CCL3 complex.33 Similarly, the CCR5 antagonist, AOP-RANTES, induces the preferential targeting of CCR5 to degradation pathways, while bioactive RANTES facilitates CCR5 recycling.6,32
Our data suggest that chemokine-Ag is efficiently delivered via clathrin-dependent chemokine receptor endocytosis to the early endosomal compartment and then is transported to late endosome and lysosome compartments, as shown for native chemokines.5,34 It is as of yet unclear whether chemokines are directly transported from endosomes to cytosol or are transported first to the lumen of the ER and then subsequently translocated into the cytosol by the pathway established for ER-associated degradation of exogenous soluble proteins and bacterial toxins.35 The involvement of the ER in the observed responses is indirectly suggested by the fact that chemokine-mediated antigen presentation was blocked by coincubation with brefeldin A, a fungal metabolite that inhibits vesicle transport between the ER and Golgi.26 On the other hand, phagosomes have been reported to display the elements and properties necessary for the lactacystin-insensitive cross-presentation of phagocytosed antigens to MHC class I molecules.36,37 Proteins were shown to be processed directly within endosomal/lysosomal compartments and loaded to MHC class I molecules, which resided in classic MHC class II compartments, using TAP-independent and NH4Cl-sensitive cross-presentation pathways.38,39 However, our data demonstrate that chemokine-mediated cross-presentation used the classic TAP-dependent MHC class I presentation pathway, since the chemokine fusion-mediated responses were completely blocked with the addition of the specific proteasomal inhibitor, lactacystin; in addition, APCs derived from TAP-1–deficient mice were unable to elicit antigen-specific T-cell activation. It has been recently postulated that DCs present antigens much more efficiently than macrophages due to lower levels of lysosomal proteases40 ; however, no notable differences were observed in our chemokine-mediated cross-presentation studies (data not shown), suggesting that the fusion-mediated processing does not involve lysosomal degradation.
Tumors express chemokines and, thus, are capable of recruiting APCs (for a review, see Proudfoot41 ), yet their antigens are not efficiently cross-presented to elicit immunity. As our data suggest, this is presumably due to the fact that the tumor antigen needs to be linked with a functionally active chemokine moiety. In support, we recently demonstrated that mice immunized with a mixture of free chemokine and tumor antigen failed to elicit antigen-specific immune responses indicating that the recruitment of APCs alone is not sufficient to induce cross-presentation.12 This is also due to the fact that most APCs, including Langerhans cells (LCs) and skin DCs, primarily deliver exogenous antigens to the MHC class II processing pathway, while cross-presentation was attributed to only CD8+ DCs (for a review, see Heath et al42 ). However, tumors may not produce the right chemokine needed for attraction of DCs, or even if they do, cross-presentation often requires large quantities of antigen (mg/mL).38,43 This is a dramatic difference with the chemokine-fused proteins that induce CD8+ T-cell responses after being cross-presented at only nanomolar (ng/mL) quantities. Typically, approaches that target various endocytic cell-surface receptors are known to increase the efficiency of antigen presentation between 100- to 10 000-fold.44,45
Cross-presentation alone is not sufficient for induction of antitumor immune responses, rather it can induce tolerance in the absence of cross-priming and other stimuli that activate costimulatory molecules B7.1 or CD4046,47 (for a review, see Heath et al42 ). In this respect, the fact that the same chemokine construct also induced CD4+ T-helper responses,13 an important requirement of cross-priming and activation of immune responses, is worth noting. Although antigens delivered to APCs without a maturation signal can also induce tolerance rather than activation of effector T cells, the fact that mice immunized with fusion protein–encoded DNA or purified fusion proteins encoding nonimmunogenic or weakly immunogenic tumor antigens in the absence of adjuvant were capable of inducing antigen-specific T-cell responses as well as protective antitumor immunity strongly supports that the necessary costimulatory pathways were being properly engaged. Chemokine fusion proteins, with the exception of murine β-defensin 2,48 have been found unable to directly induce DC maturation, although these mediators may indirectly stimulate APCs through the recruitment of proinflammatory cells or in concert with other chemokine factors, such as CCL19 and CCL21, both potent mediators facilitating the terminal activation of DCs.49
Taken together, chemokine-based vaccines are simple to generate and potent in eliciting immune responses against self-tumor or other clinically relevant weakly immunogenic antigens.11,12 The potency of the vaccines is presumably in their ability to recruit and target “important” immune cells that efficiently cross-present and cross-prime immune responses. The major limitation of the approach is in its use of the specific tumor antigens. Tumor heterogeneity in expression of particular antigens may lead to the emergence of tumor variants that fail to express the target vaccine. Moreover, although we did not detect it in mice, induction of the vaccine-induced antichemokine responses cannot be ruled out in human patients. Nevertheless, an approach to tumor vaccination using a chemokine-fused tumor antigen is worth clinical evaluation, for example, for treatment of minimal residual diseases (induced by other treatment modalities), or even as a protective vaccine to generate immune memory against tumor-specific antigens that are not expressed in normal cells and tissues.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2005-08-3207.
Supported by the Intramural Research Program of the NIH, National Institute on Aging.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Robert Pyle and Megan McCain (NIA/NIH) for technical assistance, Dr Howard Young (NCI/NIH) for the gift of the B6/129 macrophage cell line, Dr Paul Robbins (NCI/NIH) for providing gp100 cDNA and protein, Drs Dan Longo (NIA/NIH) and Ron Gress (NCI/NIH) for helpful comments and suggestions, and Ana Lustig (NIA/NIH) for critical reading of the paper.