Comment on Rosenberg et al, page 4628
In this issue of Blood, an updated analysis of the Severe Chronic Neutropenia International Registry dataset by Rosenberg and colleagues reports an alarming incidence of evolution to MDS/AML in patients who are treated with recombinant G-CSF for extended periods. We must now view congenital neutropenia (CN) as an aggressive preleukemic condition that presents with neutropenia in infants and young children, rather than as a disorder of inadequate granulocyte production. This report raises basic and translational research questions and, most importantly, demands that we reassess current treatment recommendations.
Recombinant granulocyte colony-stimulating factor (G-CSF) is a mainstay of modern therapy for congenital neutropenia (CN), a disorder characterized by chronic neutropenia, recurrent infections, and arrest at the promyelocyte/myelocyte stage of maturation (see figure). A previous analysis of CN patients who were receiving G-CSF suggested that one possible outcome is that the annual risk of evolution to myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) might rise substantially as patients received G-CSF for increasing lengths of time.1 Indeed, the careful analysis performed by Rosenberg and colleagues shows that 29% of patients who receive G-CSF for 10 years will either die from sepsis (8%) or develop MDS/AML (21%). Those who receive a daily G-CSF dose greater than 8 μg/kg yet show neutrophil counts that are below the mean for the entire group have a cumulative incidence of either fatal sepsis or myeloid leukemia of 55%. These data are stunning, even to investigators who study inherited cancer predispositions. Importantly, the myeloid malignancies that develop in patients with CN show adverse biologic features such as chromosome 7 deletions. The limited data addressing the role of hematopoietic stem cell transplantation (HSCT) in CN suggest that the outcome is poor after transformation to MDS/AML, but may cure a high proportion of patients if it is performed before any evidence of progression.FIG1
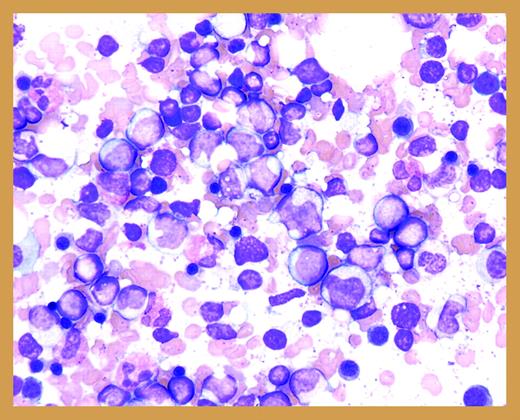
A bone marrow biopsy touch preparation from a patient with CN demonstrates increased numbers of myeloid precursors with a maturation at the promyelocyte/myelocyte stage of differentiation (courtesy of the ASH Slide Bank).
A bone marrow biopsy touch preparation from a patient with CN demonstrates increased numbers of myeloid precursors with a maturation at the promyelocyte/myelocyte stage of differentiation (courtesy of the ASH Slide Bank).
These new data also raise intriguing biologic questions. Elegant studies have elucidated an intricate signaling pathway that senses and responds to DNA damage and other types of stress, which is defective in individuals with Fanconi anemia (FA).2 By analogy, CN may fundamentally be a disorder in which the stem cell compartment is progressively depleted and acquires mutations due to an inability to respond to extracellular stress and/or to maintain genomic integrity. However, the normal sensitivity of CN cells to DNA cross-linking agents and anecdotal evidence that affected individuals tolerate standard conditioning regimens imply a distinct pathogenic mechanism in CN. How mutations in ELA2 and SBDS, the predominant causes of CN and Shwachman-Diamond syndrome, perturb the survival and stress responses of hematopoietic stem/progenitor cells is a central question for the field. A second issue involves understanding why somatic G-CSF receptor (GCSFR) mutations, which almost exclusively occur in CN, are associated with a high risk of progression to MDS/AML.
Although this new study compels us to revisit the management of patients with CN, additional molecular markers that might predict a high risk of transformation will inform clinical decision-making. For example, do specific GCSFR or ELA2 mutations predict higher rates of progression or affect the outcome of HSCT? Will myeloablative regimens be necessary to eliminate residual preleukemic cells in patients who undergo HSCT, or will these cells be eradicated or out-competed in vivo by normal donor cells? These are important questions for hematologists who have to deal with patients and their families in real time.
Although the study from the Severe Chronic Neutropenia International Registry (SCNIR) group is outstanding in many respects, I was disappointed that the investigators did not make a stronger treatment recommendation. It is important to emphasize that G-CSF remains a highly efficacious therapy to prevent serious infections.3 This potentially lifesaving treatment should not be withheld from newly diagnosed patients due to the risk of leukemia later in life. However, I believe an urgent priority is to develop more definitive guidelines in the areas of clinical monitoring, molecular testing, and how to decide whether patients with CN should remain on G-CSF or be referred for HSCT. In my opinion, the National Institutes of Health and the Leukemia and Lymphoma Society of America should convene a working meeting as soon as possible that includes clinical and laboratory investigators with expertise in the areas of stem cell and leukemia biology, HSCT, and congenital disorders of blood cell production, with the explicit goal of developing consensus treatment recommendations. Until such guidelines exist, I believe that the data are now compelling enough to recommend HSCT as the preferred treatment for patients with CN who have a matched sibling donor, and I will refer these patients to my colleagues with expertise in HSCT to consider this therapeutic option. ▪