Abstract
Increased rates of graft rejection after bone marrow transplantation (BMT) are observed in patients whose illnesses— such as sickle cell disease, thalassemia, and aplastic anemia—necessitate chronic transfusion before BMT. Because BM transplants in these patients are routinely HLA matched, any immunization responsible for increased rejection is likely against minor histocompatibility antigens (mHAs). It has been assumed that contaminating leukocytes in red blood cell (RBC) units are the main sources of immunization to mHAs. However, in this report, we demonstrate that antigens on donor RBCs are presented in the major histocompatibility complex (MHC) class I pathway of recipient antigen-presenting cells, resulting in activation and expansion of recipient CD8+ T cells specific for donor mHAs. Given that human hematopoietic progenitor cells express many of the known mHAs, this observation provides a mechanism by which chronic transfusion of even stringently leukoreduced RBCs may result in sufficient mHA immunization to increase the frequency of BMT rejection.
Introduction
Bone marrow transplantation (BMT) is now standard therapy for a variety of hematologic malignancies, and it represents a cure for certain nonmalignant hematologic disorders. To avoid the morbidity and mortality associated with stringent myeloablative therapies used to treat neoplasia, milder and less immunosuppressive conditioning regimens have been designed to treat nonmalignant illnesses. However, high rates of graft rejection are observed in patients whose illnesses—such as sickle cell disease, thalassemia, and aplastic anemia—necessitate chronic transfusion of red blood cells (RBCs) before BMT.1-3
It has been proposed that chronic transfusion immunizes the recipient to multiple donor antigens, which contributes to subsequent rejection of bone marrow grafts. Because BM transplants in patients with nonmalignant disease are routinely HLA identical, it is unlikely that immunization against major histocompatibility complex (MHC) molecules plays a role in graft rejection. However, immunization against minor histocompatibility antigens (mHAs) is known to occur in response to chronic transfusion.4 mHAs are polymorphic peptides from allelic variants of normal human proteins that are displayed by MHC molecules and elicit a specific MHC-restricted T-cell response.5
It has been assumed that contaminating leukocytes in RBC units were the main source of immunization to mHAs. Accordingly, it had been anticipated that the recent implementation of leukoreduced products would decrease the incidence of matched-related marrow graft rejection in multiply transfused patients. However, a recent analysis of patients undergoing transplantation for severe aplastic anemia, performed by the International Bone Marrow Transplant Registry, suggests that this is not the case (J.T.H., unpublished data, February 2003).
We hypothesized that RBC transfusion is sufficient to immunize against mHAs. Although exogenous antigens are typically processed and presented through MHC class II pathways, it has been reported that cell association results in a 50 000-fold greater efficiency of cross-priming into the MHC class I pathway.6 Thus, we hypothesized that donor RBC-associated antigens would be efficiently cross-presented by recipient antigen-presenting cells (APCs) into MHC class I pathways.
Study design
To crosslink chicken ovalbumin (OVA) or hen egg lysozyme (HEL) to the RBC surface, 2-pyridyldithiol groups were added to OVA (15 mg/mL) or HEL (5 mg/mL) by incubating with 0.6 mg/mL succinimidyl 6-(3-[2-pyridyldithio]-propionamido)hexanoate (LC-SPDP) (Pierce Biotechnology, Rockford IL) in PBS with 1 mM EDTA for 30 minutes, followed by the removal of unreacted LC-SPDP by dialysis. Stocks were stored at -80°C until use. RBCs from donor mice were leukoreduced, as previously described, which resulted in a 4-log10 reduction in leukocytes.7 Free sulfhydryl groups were introduced onto leukoreduced RBCs by incubation with 0.3 mg/mL of 2-imminothiolane (Traut reagent8 ; Pierce Biotechnology) for 30 minutes in bicine-saline buffer (0.02 M bicine, 0.17 M NaCl, 0.01 M NaOH, pH 8.3). LC-SPDP-modified OVA (3.8 mg/mL) or LC-SPDP-modified HEL (1.3 mg/mL) was incubated with Traut reagent-modified (Pierce Biotechnology) RBCs for 2 hours with occasional agitation and then washed with PBS.
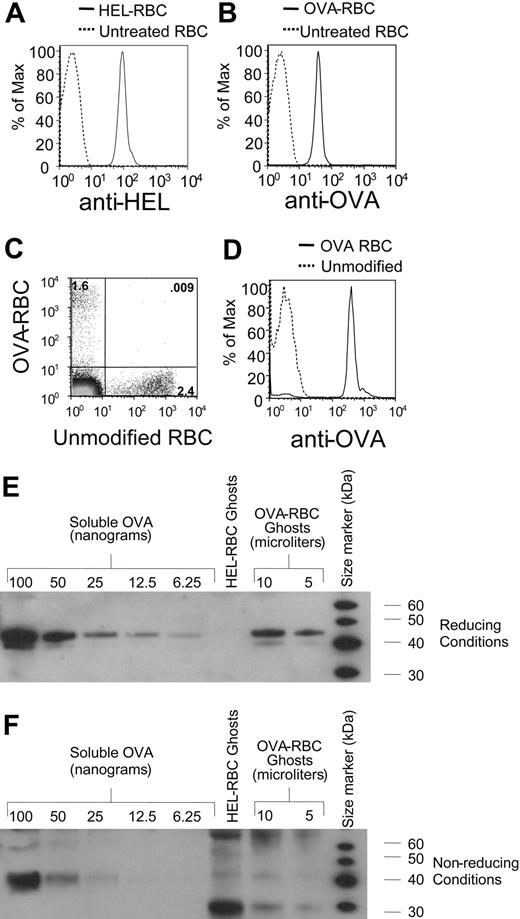
Chemical crosslinking of OVA and HEL to RBCs. OVA and HEL were crosslinked to leukoreduced RBCs, as described in “Study design.” HEL-RBCs and OVA-RBCs were stained with rabbit anti-HEL or rabbit anti-OVA, respectively (A-B). Cells were washed and incubated with goat anti-rabbit immunoglobulin conjugated to allophycocyanin. Staining of cells was analyzed by flow cytometry. OVA-RBCs were labeled with DiO, and unmodified RBCs were labeled with CM-DiI, as previously described.7 A mixture of labeled cells was transfused into C57BL/6 mice. Twenty-four hours after transfusion, peripheral blood was obtained, and the percentages of circulating RBCs were determined (C). The same blood specimen analyzed in panel C was stained with anti-OVA, as in panel B, and after appropriate gating, fluorescence was compared on transfused OVA-RBCs (solid line) compared with transfused unmodified RBCs (dashed line) (D). RBC ghosts were prepared by hypotonic lysis and were subjected to Western blot analysis with anti-OVA under reducing (E) or nonreducing (F) conditions. Standard curves of OVA were also generated using Western blots. To control for any changes in OVAimmunoreactivity as a result of the crosslinker, the OVA standard curve was generated using unreacted LC-SPDP-modified OVA.
Chemical crosslinking of OVA and HEL to RBCs. OVA and HEL were crosslinked to leukoreduced RBCs, as described in “Study design.” HEL-RBCs and OVA-RBCs were stained with rabbit anti-HEL or rabbit anti-OVA, respectively (A-B). Cells were washed and incubated with goat anti-rabbit immunoglobulin conjugated to allophycocyanin. Staining of cells was analyzed by flow cytometry. OVA-RBCs were labeled with DiO, and unmodified RBCs were labeled with CM-DiI, as previously described.7 A mixture of labeled cells was transfused into C57BL/6 mice. Twenty-four hours after transfusion, peripheral blood was obtained, and the percentages of circulating RBCs were determined (C). The same blood specimen analyzed in panel C was stained with anti-OVA, as in panel B, and after appropriate gating, fluorescence was compared on transfused OVA-RBCs (solid line) compared with transfused unmodified RBCs (dashed line) (D). RBC ghosts were prepared by hypotonic lysis and were subjected to Western blot analysis with anti-OVA under reducing (E) or nonreducing (F) conditions. Standard curves of OVA were also generated using Western blots. To control for any changes in OVAimmunoreactivity as a result of the crosslinker, the OVA standard curve was generated using unreacted LC-SPDP-modified OVA.
Results and discussion
To test our hypothesis, we generated units of leukoreduced murine RBCs that carry OVA or HEL as model antigens. Crosslinking of HEL and OVA to the RBCs was confirmed by staining with rabbit anti-HEL or rabbit anti-OVA and analyzing by flow cytometry (Figure 1A-B). To confirm that the crosslinked RBCs maintained the ability to circulate, OVA-crosslinked RBCs (OVA-RBCs) or noncrosslinked RBCs were labeled with the fluorescent dyes DiO and CM-DiI, respectively, as previously described7 and were transfused as a 1:1 mixture into recipient mice. Twenty-four hours after transfusion, recipient mice were bled, and transfused cells were visualized by flow cytometry (Figure 1C). Transfused OVA-RBCs were circulating at this time, and OVA was stable on the RBC surface (Figure 1D); however, there were fewer modified cells than unmodified cells, indicating increased clearance of modified cells. Longer-term studies revealed that OVA-RBCs have a decreased circulatory lifespan than unmodified cells but that they continue to circulate for approximately 2 weeks with stable OVA protein on the surface (data not shown).
To determine the quantity of OVA on the cell surface, RBC ghosts were prepared from OVA-RBCs and were then subjected to Western blot analysis with anti-OVA. Under reducing conditions, OVA was easily distinguished from OVA-RBCs (Figure 1E). In addition, essentially all the observed OVA was covalently bound to the RBCs by disulfide bonds because only a very weak, nonspecific band was observed with the molecular weight of OVA under nonreducing conditions (Figure 1F). A titration of soluble OVA demonstrated that 10 μL OVA-RBC ghosts contained approximately 50 ng OVA (Figure 1E). Multiplying this amount by the volume of ghosts recovered from a given volume of OVA-RBCs revealed that 100 μL packed OVA-RBCs contained approximately 1 μg OVA protein.
The OT-I mouse is transgenic for a T-cell receptor (TCR) that is specific for OVA peptide 257-264 (SIINFEKL) presented by MHC class I H-2Kb.9 Thus, activation and expansion of OT-I T cells provides a readout for presentation of this OVA peptide. Splenocytes from OT-I mice were adoptively transferred into C57BL/6 mice congenic for Thy 1.1 (B6.Thy 1.1 mice). One day later, the mice received a transfusion of 100 μL OVA-RBCs, HEL-RBCs, soluble OVA, or no transfusion. Five animals were included in each group that received transfusions. B10.BR (H-2k) mice were used as RBC donors. Because B10.BR mice do not express H-2Kb, this experimental design precludes the possibility that any observed expansion of the OT-I T cells was caused by direct presentation of OVA by donor cells.
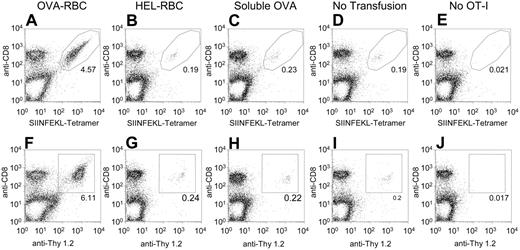
Three days after the transfusion, splenocytes were isolated from the recipient animals, and OT-I T cells were visualized by staining with anti-CD8 and a tetramer specific for the OT-I TCR (SIINFEKL-Tetramer) (Figure 2A-E; Table 1). In mice that received OVA-RBCs, OT-I T cells expanded to a total number of 4.4 million cells, which represents a 49-fold expansion of OT-I T cells compared with mice that received no transfusion (P = .0002). In contrast, HEL-RBCs resulted in a small (2-fold) expansion (P = .04), and no significant expansion was seen for soluble OVA (1.3-fold; P = .07). The observed expansion was not dependent on the antigen being linked by disulfide bonds because the same experiment using an irreversible maleimide-based crosslinker (sulfosuccinimidyl 4-[p-maleimidophenyl]butyrate) gave similar results (data not shown). Because TCRs can be down-modulated on activation, staining with anti-CD8 and anti-Thy 1.2 was also used as a separate measure of OT-I T cells. Staining with anti-Thy 1.2 demonstrated the same trend, but with a slightly greater increase of OT-I T cells in the OVA-RBC-transfused mice (Figure 2F-J).
Expansion of OT-I T cells in response to transfusion of OVA-RBCs. OT-I splenocytes (20 × 106) were adoptively transferred into B6.Thy1.1 mice. One day later, mice underwent transfusion with 100 μL OVA-RBCs, 100 μL HEL-RBCs, or soluble OVA. One microgram soluble OVA was used because this was the amount of OVA contained on 100 μL OVA-RBCs (see Figure 1). To control for any changes in OVA immunoreactivity as a result of the crosslinker, the soluble OVA used was LC-SPDP-modified OVA. There were 5 animals in each experimental group; 2 animals in the control group received no transfusions. Three days after transfusion, splenocytes were isolated from recipient animals, and OT-I T cells were visualized by staining with anti-CD8 and tetramer (A-E) or anti-CD8 and anti-Thy 1.2 (F-J). The legitimacy of the gates used to identify OT-I cells was confirmed by staining splenocytes from mice that had received no OT-I T cells (E,J). Total numbers of OT-I T cells were calculated by counting the number of splenocytes recovered from each animal and multiplying by the resulting percentages of CD8+ tetramer+ cells. Representative flow cytometry plots from individual animals are presented (A-J). Five animals were included in each group that underwent transfusion, and the numerical averages for each group are presented in Table 1. Standard deviations were calculated by combining the results of all animals in a given group using total numbers of cells. Probabilities were determined as 2-tailed values generated using an unpaired t test. This experiment was performed 4 times with similar results.
Expansion of OT-I T cells in response to transfusion of OVA-RBCs. OT-I splenocytes (20 × 106) were adoptively transferred into B6.Thy1.1 mice. One day later, mice underwent transfusion with 100 μL OVA-RBCs, 100 μL HEL-RBCs, or soluble OVA. One microgram soluble OVA was used because this was the amount of OVA contained on 100 μL OVA-RBCs (see Figure 1). To control for any changes in OVA immunoreactivity as a result of the crosslinker, the soluble OVA used was LC-SPDP-modified OVA. There were 5 animals in each experimental group; 2 animals in the control group received no transfusions. Three days after transfusion, splenocytes were isolated from recipient animals, and OT-I T cells were visualized by staining with anti-CD8 and tetramer (A-E) or anti-CD8 and anti-Thy 1.2 (F-J). The legitimacy of the gates used to identify OT-I cells was confirmed by staining splenocytes from mice that had received no OT-I T cells (E,J). Total numbers of OT-I T cells were calculated by counting the number of splenocytes recovered from each animal and multiplying by the resulting percentages of CD8+ tetramer+ cells. Representative flow cytometry plots from individual animals are presented (A-J). Five animals were included in each group that underwent transfusion, and the numerical averages for each group are presented in Table 1. Standard deviations were calculated by combining the results of all animals in a given group using total numbers of cells. Probabilities were determined as 2-tailed values generated using an unpaired t test. This experiment was performed 4 times with similar results.
Together, the data presented herein indicate that RBC-associated proteins undergo cross-presentation by recipient APCs into MHC class I pathways and that this results in the functional activation and expansion of mHA-specific CD8+ T cells. Human hematopoietic progenitor cells express many of the known mHAs. Thus, any protein expressed by RBCs that is also expressed in hematopoietic cells can serve as an mHA, for which an immune response may be primed by RBC transfusion and may lead to increased rejection of the BM transplant. The observation that donor RBC antigens can be presented by MHC class I of host APCs and can stimulate host CD8+ T cells provides a mechanism by which chronic transfusion of even stringently leukoreduced RBCs may result in sufficient immunization to mHA transplantation antigens to cause an increased frequency of BMT rejection.
Prepublished online as Blood First Edition Paper, August 25, 2005; DOI 10.1182/blood-2005-07-3059.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Traci Chadwick for outstanding technical assistance.