Abstract
Mammalian nonheme iron absorption requires reduction of dietary iron for uptake by the divalent metal ion transport system in the intestine. This was thought to be mediated by duodenal cytochrome b (Cybrd1), a ferric reductase enzyme resident on the luminal surface of intestinal absorptive cells. To test its importance in vivo, we inactivated the murine Cybrd1 gene and assessed tissue iron stores in Cybrd1-null mice. We found that loss of Cybrd1 had little or no impact on body iron stores, even in the setting of iron deficiency. We conclude that other mechanisms must be available for the reduction of dietary iron. (Blood. 2005;106: 2879-2883)
Introduction
Iron is an essential element that must be extracted from the diet. Dietary nonheme iron is generally found in the ferric (Fe3+) oxidation state. It must be reduced to ferrous (Fe2+) ion to be transported by divalent metal ion transporter 1 (DMT1, also known as Slc11a2), the predominant transmembrane iron carrier on the apical surface of the intestinal mucosa.1 The reduction of dietary iron is thought to be mediated by an enzymatic ferric reductase in the intestinal brush border.2 Ferric reductase activities dependent upon ascorbate, nicotinamide adenine dinucleotide (NADH), and nicotinamide adenine dinucleotide phosphate (NADPH) have been described in yeast,3 plants,4 and mammalian cell lines,5-7 but the molecular identity of the mammalian intestinal ferric reductase has been uncertain.
Using a subtractive cloning strategy, McKie et al8 isolated a compelling candidate, called Dcytb (Cybrd1) for duodenal cytochrome b. Cybrd1 is a putative plasma membrane diheme protein that is induced in mouse duodenal mucosa under conditions of accelerated intestinal iron absorption including hypotransferrinemia, iron deficiency, and hypoxia. It is also induced in pregnancy and after induction of hemolytic anemia by administration of phenylhydrazine.9,10 It is situated on the brush-border membrane of mature duodenal enterocytes, and it confers ferric reductase activity when expressed in Xenopus oocytes or cultured mammalian cells.8 Taken together, these results strongly suggested that Cybrd1 was an important component of the intestinal iron absorption machinery. Additional, supportive evidence for its role came from studies that demonstrated that Cybrd1 mRNA is increased in animals with genetic hemochromatosis.11,12
Cybrd1 is not homologous to previously described ferric reductases from lower eukaryotes.8 Its closest homolog is mammalian cytochrome b561, an enzyme with no apparent role in iron homeostasis. Like cytochrome b561, Cybrd1 has 6 predicted transmembrane segments and 4 conserved histidine residues that have been proposed to serve as heme ligands. Both proteins lack apparent NADH-, NADPH-, or flavin-binding motifs that would allow these cofactors to act as electron donors. Cytochrome b561 receives an electron from intracellular ascorbate. Others have postulated that Cybrd1 may also use ascorbate or, analogous to the mammalian enzyme gp91phox, associate with several other proteins to form an active complex.8
In spite of compelling evidence for a critical role for Dcytb in intestinal iron absorption, its function had not previously been evaluated in vivo. To develop a model for study, we disrupted murine Cybrd1 by gene targeting. We found that the absence of Cybrd1 did not impair accumulation of body iron stores when mice were fed normal chow, indicating that there was no major effect on intestinal absorption. Loss of Cybrd1 had a mild effect, at most, on tissue iron content in mice fed an iron-deficient diet. We conclude that Cybrd1 is not an essential component of the intestinal iron absorption apparatus in mice.
Materials and methods
Targeted disruption of the murine Cybrd1 gene
We isolated Cybrd1 genomic DNA clones from a strain 129 mouse library (Stratagene, La Jolla, CA). To construct the vector for inactivation of Cybrd1, we subcloned genomic DNA into a pTKLNC targeting vector and introduced a loxP site into intron 1 and a loxP-flanked (floxed) neomycin-resistance cassette into intron 2. Details of vector construction are available upon request. The targeting vector was linearized by digestion with NcoI and electroporated into 129 J1 embryonic stem (ES) cells. The neomycin-resistant cassette was subsequently excised by transient expression of Cre recombinase as described previously.1 Correct homologous recombination and Cre-mediated excision were confirmed by Southern blot analysis. ES cell clones with normal karyotypes were injected into C57BL/6 blastocysts. Transmission of the targeted alleles was confirmed by Southern blot analysis. Subsequent genotyping was carried out by Southern blot and/or polymerase chain reaction (PCR) analysis on DNA extracted from snipped tail samples (PureGene kit; Gentra Systems, Minneapolis, MN). Detailed probe and primer information are available upon request.
Animal care
All mice were born and housed in the barrier facility at Children's Hospital Boston. All mouse procedures were approved by and in compliance with guidelines of the Institutional Animal Care and Use Committee at Children's Hospital Boston. Female mice were analyzed unless otherwise noted. We weaned pups at 28 days and maintained them on Prolab RMH 3000 LabDiet (PMI Richmond, Richmond, IN), which has 380 parts per million (ppm) iron, or iron-deficient diet (TD 80396; Harlan Teklad, Madison, WI) containing less than 5 ppm iron. We administered deionized water (milliQ; Millipore, Billerica, Spain) to mice fed the iron-deficient diet. A vitamin mixture (Vitamin Mix, AIN-76A; Harlan Teklad, Madison, WI) was included in the diet.
Iron assays
We assayed serum iron concentrations using a serum iron/unsaturated iron binding capacity (UIBC) kit (ThermoDMA, East Arlington, TX) according to the manufacturer's instructions. We determined nonheme liver iron concentrations as previously described.13
Cybrd1 antibody
A rabbit anti-Cybrd1 antibody was raised against the peptide DAESSSEGAARDRTLGLADSGQRSTM, which corresponds to the C-terminal 26 amino acids (265-290) of the murine protein. The peptide was coupled to keyhole limpet hemocyanin by the addition of an N-terminal cysteine residue. Specific antibody was subsequently purified on a peptide affinity column (Sigma Genosys, The Woodlands, TX).
Enterocyte preparation and immunoblot analysis
Enterocyte lysates were prepared from intestinal specimens and immunoblotted as previously described.1 Trf hpx/hpx, Cybrd1-/-, and wild-type mice were 12 weeks old. Cybrd1-/- and wild-type mice were fed an iron-deficient diet for 8 weeks prior to the analysis. Immunoblotting was carried out using the purified anti-Cybrd1 antibody or anti-Slc11a2 antiserum (1:3000, from Philippe Gros).
RNA preparation
Duodenum and liver samples were harvested from Trf hpx/hpx, Cybrd1-/-, and wild-type mice and immediately stored in RNAlater (Ambion, Austin, TX). Total RNA was isolated using RNA STAT-60 (Leedo Medical Laboratories, Houston, TX) according to the manufacturer's instructions.
Northern blot analysis
We used 10 μg total RNA from each mouse and ran a 1% formaldehyde agarose gel. Then we blotted it onto a positively charged Nylon membrane, Hybond-N+ (Amersham, Little Chalfont, United Kingdom). We hybridized the membrane to digoxigenin-labeled probes specific for Slc40a1 (ferroportin), Hamp (hepcidin), and actin mRNAs. We detected the signals by exposing Biomax MR film (Kodak, Rochester, NY).
Results
Targeted disruption of Cybrd1
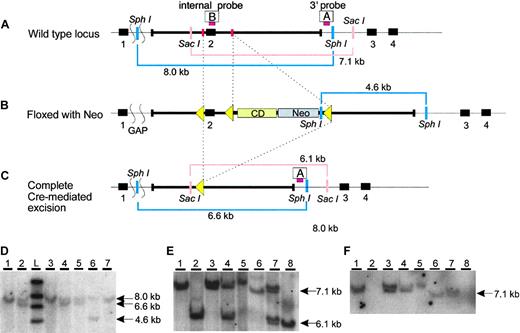
The murine Cybrd1 gene consists of 4 exons on chromosome 2. The putative binding sites for cofactors are encoded by the second exon.8 Taking this into account, we designed a targeting construct to delete exon 2 (Figure 1A-C). This construct was introduced into mouse ES cells and homologous recombination was confirmed by Southern blot analysis of both ends of the recombined segment (using the 3′-probe shown in Figure 1D; data using the 5′-probe not shown). The targeted allele was introduced into the mouse germ line and maintained on a homogeneous 129S6/SvEvTac inbred background (Figure 1E). We confirmed that exon 2 was successfully deleted in mice homozygous for the targeted allele (Cybrd1-/-;Figure 1F).
Cybrd1 is not essential for dietary iron absorption
Cybrd1-/- mice had no visible phenotype. When fed a normal diet, there was no evidence of functional iron deficiency. The mice were not anemic, and their hematologic parameters were indistinguishable from wild-type mice (not shown). We evaluated liver nonheme iron content as a surrogate for total body iron stores. There was a trend toward lower tissue iron levels in Cybrd1-/- mice fed a normal diet, but the differences did not reach statistical significance (Figure 2A).
Previously, others had demonstrated induction of Cybrd1 in response to iron deficiency. We speculated that Cybrd1 might not be necessary under normal husbandry conditions but might aid in iron absorption under stress. To test this, we maintained the mice on an iron-deficient diet for 8 weeks after weaning.
We confirmed that this regimen induced iron deficiency in both wild-type and Cybrd1-/- mice. Although neither group developed anemia, the body weights and tissue iron concentrations in mice of both genotypes were significantly lower than those of mice fed a normal diet (Table 1). We noted a trend toward lower iron stores in iron-deficient Cybrd1-/- mice compared with iron-deficient wild-type mice, but the difference did not reach statistical significance. The difference in mean liver iron concentrations at 12 weeks of age appeared larger than the difference in weanling mice (Figure 2A), suggesting that the difference might become significant if the mice remained on an iron-deficient diet for a substantially longer period of time.
Comparing iron-deficient, 12-week-old wild-type and Cybrd1-/- mice, we saw no significant differences in either the body weights (Table 1) or the liver weights (0.701 ± 0.028 g in wild-type versus 0.631 ± 0.049 g in Cybrd1-/- mice, P = .218). However, the ratio of the liver weight relative to total body weight was greater in wild-type mice than in Cybrd1-/- mice (wild type, 0.0353 ± 0.001 versus Cybrd1-/-, 0.0311 ± 0.001; P = .009). Accordingly, when we normalized liver nonheme iron content by liver and body weights, the relative liver iron content in iron-deficient Cybrd1-/- mice was significantly lower (Figure 2B).
To confirm that no Cybrd1 protein was expressed in Cybrd1-/- mice, we developed a specific anti-Cybrd1 antiserum that recognizes the protein on immunoblots. The specificity of the antibody was confirmed by comparing immunoblots from cells that were or were not transfected with a Cybrd1 expression construct (not shown). In wild-type duodenal lysates, we typically observed 2 specific bands, with apparent molecular weights of 30 to 35 kDa and 60 to 70 kDa (Figure 2C). The faster migrating band is consistent with the predicted mass of Cybrd1. The larger band was not detected using preimmune serum and was not seen in tissues that did not express Cybrd1. We speculate that it represents a Cybrd1 dimer that has not denatured under the conditions used to prepare the blot. Cybrd1 protein was highly expressed in duodenal lysates from anemic Trf hpx/hpx mice (Figure 2C), as reported previously.8 It was readily detectable in samples from wild-type mice. However, no Cybrd1 protein was detected in duodenal lysates from Cybrd1-/- mice (Figure 2C,E). We observed a large increase in Cybrd1 protein expression in lysates from wild-type mice maintained on an iron-deficient diet for 27 weeks compared with wild-type mice fed a regular diet (Figure 2E).
Targeted disruption of Cybrd1. (A) Cybrd1 wild-type locus and (B) targeted locus after introduction of an intronic floxed neomycin (Neo)/cytosine deaminase (CD) cassette and loxP sites (yellow triangles). Positions of 3′ and internal probes used for Southern blot analysis are shown as red bars A and B, respectively. (C) Cybrd1 locus after complete excision to inactivate the gene. Squiggled lines to left of diagrams in A-C indicate segments of DNA not included in the figure. Pink bars indicate the size of fragments digested by SacI using a 3′ probe by Southern analysis, and blue bars indicate the length of fragments digested by SphI using a 5′ probe by the same analysis. (D) Southern blot analysis of the 3′ end of the SphI digested locus in ES cells after Cre-mediated excision, using probe A. Clone 6 retains the floxed allele; clones 2, 4, and 5 have undergone complete excision, deleting exon 2. Clones 2 and 4 were used to obtain Cybrd1-/- mice without residual Neo and CD cassettes. (E-F) Southern blot analysis of the 3′ end of the SacI digested locus in genomic DNA using probe A (E), and the internal probe B within exon 2 (F). Lanes 1, 3, 5, and 6 show wild-type mice, 2 and 8 show homozygous Cybrd1-/- mice, and 4 and 7 show heterozygous Cybrd1-/+ mice.
Targeted disruption of Cybrd1. (A) Cybrd1 wild-type locus and (B) targeted locus after introduction of an intronic floxed neomycin (Neo)/cytosine deaminase (CD) cassette and loxP sites (yellow triangles). Positions of 3′ and internal probes used for Southern blot analysis are shown as red bars A and B, respectively. (C) Cybrd1 locus after complete excision to inactivate the gene. Squiggled lines to left of diagrams in A-C indicate segments of DNA not included in the figure. Pink bars indicate the size of fragments digested by SacI using a 3′ probe by Southern analysis, and blue bars indicate the length of fragments digested by SphI using a 5′ probe by the same analysis. (D) Southern blot analysis of the 3′ end of the SphI digested locus in ES cells after Cre-mediated excision, using probe A. Clone 6 retains the floxed allele; clones 2, 4, and 5 have undergone complete excision, deleting exon 2. Clones 2 and 4 were used to obtain Cybrd1-/- mice without residual Neo and CD cassettes. (E-F) Southern blot analysis of the 3′ end of the SacI digested locus in genomic DNA using probe A (E), and the internal probe B within exon 2 (F). Lanes 1, 3, 5, and 6 show wild-type mice, 2 and 8 show homozygous Cybrd1-/- mice, and 4 and 7 show heterozygous Cybrd1-/+ mice.
As we previously reported, Slc11a2 protein levels were also markedly increased in Trf hpx/hpx duodenal lysates (Figure 2D-E).1 There was at most a slight increase in Slc11a2 protein expression in wild-type mice fed an iron-deficient diet for 8 weeks (Figure 2D) or 27 weeks (Figure 2E). However, levels remained much lower than those observed in Trf hpx/hpx duodenal lysates. Slc11a2 protein expression was not induced in iron-deficient Cybrd1-/- duodenal lysates compared with iron-deficient wild-type duodenal lysates (Figure 2D-E). No Slc40a1 protein expression was detectable in iron-deficient Cybrd1-/- or wild-type duodenal lysates (Figure 2E), though Slc40a1 protein was highly expressed in duodenal lysates from anemic Trf hpx/hpx mice (Figure 2E) as reported previously.14
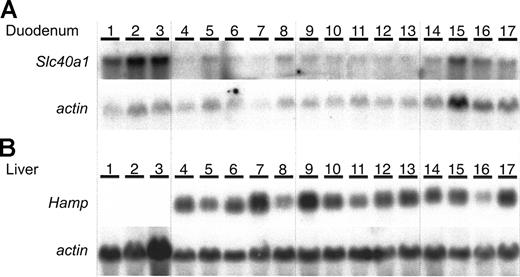
Because we could not detect Slc40a1 protein, we used Northern blot analysis to examine levels of Slc40a1 mRNA (Figure 3A). Slc40a1 mRNA levels were markedly increased in Trf hpx/hpx duodenum (Figure 3A) as reported previously.14 However, Slc40a1 mRNA levels in iron-deficient Cybrd1-/- duodenum were indistinguishable from those observed in iron-deficient wild-type duodenum (Figure 3A). Furthermore, there was no significant difference in liver Hamp mRNA expression levels between Cybrd1-/- and wild-type mice (Figure 3B). Hamp mRNA levels were markedly decreased in samples of Trf hpx/hpx duodenum, as reported previously.15
Discussion
We have previously shown that absorption of dietary nonheme iron is highly dependent upon the activity of Slc11a2, a transmembrane Fe2+ transporter.1 Slc11a2 will not accept Fe3+ as a substrate.16 Since iron is readily oxidized in an oxygen-rich, neutral pH environment, and iron in food is predominantly Fe3+, there must be a mechanism for reducing iron for intestinal uptake. Cybrd1, a ferric reductase resident in the brush border, was a likely candidate to carry out this function. Its expression has been reported to be regulated in response to changes in body iron status.
Because the function of Cybrd1 had not been evaluated in vivo, we disrupted murine Cybrd1 by gene targeting and analyzed the phenotypes of Cybrd1-/- mice. We show here that inactivation of the Cybrd1 gene has little, if any, effect on the body's ability to accumulate tissue iron stores, even in the setting of iron deficiency. Furthermore, expression patterns of other genes involved in intestinal iron absorption, including Slc11a2, Slc40a1, and Hamp, were indistinguishable when we compared wild-type and Cybrd1-/- mice maintained on an iron-deficient diet.
Liver iron loading and duodenal protein expression in mice with Cybrd1 mutations. (A) There was slightly less nonheme liver iron content, on average, in Cybrd1-/- animals compared with wild-type animals (12-week-old males: wild type [n = 3], Cybrd1-/- [n = 3]; 4-week-old females: wild type [n = 9], Cybrd1-/- [n = 7]; 12-week-old females: wild type [n = 10], Cybrd1-/- [n = 9]). The differences did not reach statistical significance, but the trend was greatest in female mice maintained on an Fe(-) (iron deficient) diet. Values are shown as a percentage of the values seen in wild-type mice. (B) Liver nonheme iron content (calculated for whole liver and adjusted for body weight) in 12-week-old female mice fed an iron-deficient diet for 8 weeks (n = 9-10). Results in μg/g wet weight are expressed as mean ± SEM. Statistical analysis was performed using the unpaired Student t test, comparing mutant versus wild-type mice, P = .019. (C-D) Immunoblot analysis of duodenal lysates from Trfhpx/hpx (lanes 1 and 7), iron-deficient wild-type (lanes 2-3), and iron-deficient Cybrd1-/- (lanes 4-5) mice using anti-Cybrd1 antiserum (C) or anti-Slc11a2 antiserum (D). The single band shown in panel D ran between the 60-kDa and 85-kDa markers. No sample was loaded in lane 6. Protein amounts loaded per lane were 10 μg wild-type and Cybrd1-/- lysate, and 2 μg (lane 1) and 4 μg (lane 7) Trfhpx/hpx duodenal lysate. (E) Immunoblot analysis of duodenal lysates from Trfhpx/hpx mice (lanes 1-2), a Cybrd1-/- mouse maintained on an iron-deficient diet for 27 weeks (lane 3), a wild-type mouse maintained on an iron-deficient diet for 27 weeks (lane 4), and a wild-type mouse on a regular iron diet (lane 5) using anti-Slc11a2 antiserum, anti-Cybrd1 antibody, or anti-Slc40a1 antibody.
Liver iron loading and duodenal protein expression in mice with Cybrd1 mutations. (A) There was slightly less nonheme liver iron content, on average, in Cybrd1-/- animals compared with wild-type animals (12-week-old males: wild type [n = 3], Cybrd1-/- [n = 3]; 4-week-old females: wild type [n = 9], Cybrd1-/- [n = 7]; 12-week-old females: wild type [n = 10], Cybrd1-/- [n = 9]). The differences did not reach statistical significance, but the trend was greatest in female mice maintained on an Fe(-) (iron deficient) diet. Values are shown as a percentage of the values seen in wild-type mice. (B) Liver nonheme iron content (calculated for whole liver and adjusted for body weight) in 12-week-old female mice fed an iron-deficient diet for 8 weeks (n = 9-10). Results in μg/g wet weight are expressed as mean ± SEM. Statistical analysis was performed using the unpaired Student t test, comparing mutant versus wild-type mice, P = .019. (C-D) Immunoblot analysis of duodenal lysates from Trfhpx/hpx (lanes 1 and 7), iron-deficient wild-type (lanes 2-3), and iron-deficient Cybrd1-/- (lanes 4-5) mice using anti-Cybrd1 antiserum (C) or anti-Slc11a2 antiserum (D). The single band shown in panel D ran between the 60-kDa and 85-kDa markers. No sample was loaded in lane 6. Protein amounts loaded per lane were 10 μg wild-type and Cybrd1-/- lysate, and 2 μg (lane 1) and 4 μg (lane 7) Trfhpx/hpx duodenal lysate. (E) Immunoblot analysis of duodenal lysates from Trfhpx/hpx mice (lanes 1-2), a Cybrd1-/- mouse maintained on an iron-deficient diet for 27 weeks (lane 3), a wild-type mouse maintained on an iron-deficient diet for 27 weeks (lane 4), and a wild-type mouse on a regular iron diet (lane 5) using anti-Slc11a2 antiserum, anti-Cybrd1 antibody, or anti-Slc40a1 antibody.
There are several caveats to the conclusion that Cybrd1 is dispensable in mammals. First, we have investigated its importance in only one inbred mouse strain. The 129S6/SvEvTac mice used in our experiments are known to have larger tissue iron stores than other commonly used laboratory strains (J. Levy and N.C.A., unpublished results, July 1999). The 129S6/SvEvTac mice do not become anemic on an iron-deficient dietary regimen that induces anemia in other strains. It remains possible that they are unique in having a redundant enzymatic mechanism for duodenal iron reduction. Second, it is possible that Cybrd1 is not necessary for iron absorption in mice, but is still important in other species. Mice are able to produce endogenous ascorbate, unlike humans and certain other species that have lost an enzyme needed in the last step of ascorbate synthesis. Ascorbate was presumed to function as a cofactor for Cybrd1 to effect iron reduction,17 but it is possible that ascorbate secreted into the intestinal lumen might itself have served as a reducing agent. Third, it is possible that antioxidants present in the chow could serve as reductants to nonenzymatically reduce Fe3+ to Fe2+.
Northern blot analysis in mice with Cybrd1 mutations. (A) Duodenal Slc40a1 mRNA expression and (B) liver Hamp mRNA expression. Samples were compared from Trfhpx/hpx mice (lanes 1-3), iron-deficient Cybrd1-/- mice (lanes 4-8), iron-deficient wild-type mice (lanes 9-13), and wild-type mice on normal diet (lanes 14-17). The identification numbers represent the same animals in panels A and B.
Northern blot analysis in mice with Cybrd1 mutations. (A) Duodenal Slc40a1 mRNA expression and (B) liver Hamp mRNA expression. Samples were compared from Trfhpx/hpx mice (lanes 1-3), iron-deficient Cybrd1-/- mice (lanes 4-8), iron-deficient wild-type mice (lanes 9-13), and wild-type mice on normal diet (lanes 14-17). The identification numbers represent the same animals in panels A and B.
We favor the idea that at least one other ferric reductase enzyme can function in dietary iron absorption. Other proteins related to Cybrd1 have been shown to have ferric reductase activity, though they have not previously been implicated in intestinal iron uptake.18 Unrelated intestinal ferric reductases may exist but have not yet been described. It may be that Cybrd1 acts as the primary duodenal reductase, but that other, redundant activities readily assume its function when it is absent. Regardless, our results suggest that Cybrd1 is not as important in murine intestinal iron transport as it was presumed to be.
The most compelling evidence for an important role of Cybrd1 in iron metabolism was its regulation in response to conditions associated with increased intestinal iron absorption8 and its increased expression in murine hemochromatosis.11,12 Cybrd1 is highly conserved across mammalian species. We must now consider the possibility that the pattern of regulation and evolutionary conservation relate to a different, as-yet-unknown function for Cybrd1. However, if Cybrd1 does have another function, it does not appear to be essential for viability or normal mouse development.
Prepublished online as Blood First Edition Paper, June 16, 2005; DOI 10.1182/blood-2005-02-0716.
Supported by National Institutes of Health (NIH) grants R01 DK53813 and R01 DK066373 to N.C.A., who is also an investigator of the Howard Hughes Medical Institute. H.G. was supported by an American Gastroenterology Association (AGA)/American Digestive Health Foundation (ADHF)/Foundation of Digestive Health and Nutrition (FDHN) Research Scholar Award and NIH K01 DK02804. M.D.F. is supported, in part, by NIH R01 DK 062474 and R01 DK 066373.
H.G. designed and participated in the execution of all experiments described in this report and contributed to writing the paper. H.G., C.N.S., and V.M.S. initially designed and prepared the Cybrd1 gene-targeting construct. M.D.F. and E.L.G. produced and characterized anti-Cybrd1 antiserum. J.J. assisted with detection of Cybrd1 and Slc11a2 proteins in enterocytes. C.D. and S.M.G. provided technical assistance. N.C.A. guided the overall development of the research plan and contributed to writing the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
ES cell manipulations and blastocyst injections were carried out by the Mental Retardation Research Center Gene Manipulation Facility at Children's Hospital, funded in part by NIH P30 HD18655. We thank Philippe Gros for his generous gift of anti-Slc11a2 antiserum, Adriana Donovan for her gift of anti-Slc40a1 antibody, and Vonnie Lee for technical assistance. We also appreciate the help of other members of the Fleming and Andrews laboratories. The authors have no conflicts of interest to report.

![Figure 2. Liver iron loading and duodenal protein expression in mice with Cybrd1 mutations. (A) There was slightly less nonheme liver iron content, on average, in Cybrd1-/- animals compared with wild-type animals (12-week-old males: wild type [n = 3], Cybrd1-/- [n = 3]; 4-week-old females: wild type [n = 9], Cybrd1-/- [n = 7]; 12-week-old females: wild type [n = 10], Cybrd1-/- [n = 9]). The differences did not reach statistical significance, but the trend was greatest in female mice maintained on an Fe(-) (iron deficient) diet. Values are shown as a percentage of the values seen in wild-type mice. (B) Liver nonheme iron content (calculated for whole liver and adjusted for body weight) in 12-week-old female mice fed an iron-deficient diet for 8 weeks (n = 9-10). Results in μg/g wet weight are expressed as mean ± SEM. Statistical analysis was performed using the unpaired Student t test, comparing mutant versus wild-type mice, P = .019. (C-D) Immunoblot analysis of duodenal lysates from Trfhpx/hpx (lanes 1 and 7), iron-deficient wild-type (lanes 2-3), and iron-deficient Cybrd1-/- (lanes 4-5) mice using anti-Cybrd1 antiserum (C) or anti-Slc11a2 antiserum (D). The single band shown in panel D ran between the 60-kDa and 85-kDa markers. No sample was loaded in lane 6. Protein amounts loaded per lane were 10 μg wild-type and Cybrd1-/- lysate, and 2 μg (lane 1) and 4 μg (lane 7) Trfhpx/hpx duodenal lysate. (E) Immunoblot analysis of duodenal lysates from Trfhpx/hpx mice (lanes 1-2), a Cybrd1-/- mouse maintained on an iron-deficient diet for 27 weeks (lane 3), a wild-type mouse maintained on an iron-deficient diet for 27 weeks (lane 4), and a wild-type mouse on a regular iron diet (lane 5) using anti-Slc11a2 antiserum, anti-Cybrd1 antibody, or anti-Slc40a1 antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/8/10.1182_blood-2005-02-0716/6/m_zh80200585170002.jpeg?Expires=1769106042&Signature=AOI-7ZLEmpJ7b-BU2lRHBvZ15VTh5gf~9CVbGa6TDK7QiJK68vRSuGBGqY9rIXenYvU1I-7eekIWxxfhsqiRxouoJumk6fcg73mixU-oWARTwbaEXgwrhJwV92SkeVKv2uc0pXEZEXR2K8rSHUYpaMDpBIcS8Q~~r9fzxfbBm0E7aiH3g4E6uD~H1MdYOv6KtjDcB9fttFw14SkKm53pTIK6BzeszdKgno5VI4f4kbyUoRRnUd9x1Htgb2t1iAYSpzBBh~a3sUVJ-WKRzaYBtMvt-x7qd5S0gYFQyvSy-Jptlmta~GqKsgGKLFRZYAdBZSkzaek5QcPnKFIx2ucong__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)