Abstract
Although studies have shown increased evidence of death receptor-driven apoptosis in intestinal lymphoid cells, splenocytes, and the liver following the onset of polymicrobial sepsis, little is known about the mediators controlling this process or their pathologic contribution. We therefore attempted to test the hypothesis that the hydrodynamic administration of small interfering RNA (siRNA) against the death receptor, Fas or caspase-8, should attenuate the onset of morbidity and mortality seen in sepsis, as produced by cecal ligation and puncture (CLP). We initially show that in vivo administration of green fluorescent protein (GFP) siRNA in GFP transgenic mice results in a decrease in GFP fluorescence in most tissues. Subsequently, we also found that treating septic nontransgenic mice with siRNA targeting Fas or caspase-8 but not GFP (used as a control here) decreased the mRNA, in a sustained fashion up to 10 days, and protein expression of Fas and caspase-8, respectively. In addition, transferase-mediated dUTP (deoxyuridine triphosphate) nick end labeling (TUNEL) and active caspase-3 analyses revealed a decrease in apoptosis in the liver and spleen but not the thymus following siRNA treatment. Indices of liver damage were also decreased. Finally, the injection of Fas or caspase-8 given not only 30 minutes but up to 12 hours after CLP significantly improved the survival of septic mice.
Introduction
Sepsis affects approximately 750 000 people in the United States every year, and one third of the reported cases result in death.1 Common causes include traumatic injury, severe bacterial infections, or severe burns. However, sepsis also frequently affects critically ill, elderly, pediatric, and postsurgical patients in the intensive care unit. With the exception of the recent application of activated protein C, most molecular-biologic-based therapies have failed clinically. Treatment using antibiotics mildly reduces risk of death; however, it is ineffective on mice with severe sepsis, as measured by high inflammatory cytokine levels.2 Thus, there is an urgent need not only for better understanding of the pathology of sepsis and its resultant organ failure, but also for new therapeutic approaches and targets.
We and other laboratories have previously reported that organ damage and mortality associated with sepsis in mouse models is at least in part due to the activation of the Fas-FasL (Fas ligand) signaling pathway, and not Toll-like receptor 4 (TLR4).3 Studies using Fas fusion protein given 12 hours after cecal ligation and puncture (CLP) show a protective effect against apoptosis in Kupffer cells, which seems to benefit the liver in a way that reduces organ damage and, in turn, improves survival after sepsis.4 This blockade of FasL also restores total hepatic, intestinal, and cardiac blood flow while attenuating the plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), indicating reduced liver damage.5 In addition, studies using FasL-/- mice provide further evidence for death receptor-mediated apoptosis in sepsis, because FasL-/- mice also show a marked reduction in septic mortality.3 Nonetheless, little is known about whether truncation of FasL downstream signaling events are protective against septic morbidity and mortality, because few or no studies have examined these signaling targets in sepsis. Pharmacologic inhibitors of caspases such as caspase-8 or caspase-3 have shown some protection, specifically with pan-specific caspase inhibition.6 However, significant considerations must be made with caspase peptide inhibitors not only to avoid toxicity and maintain a protective dosage but also to retain caspase specificity. Alternatively, there is a void of inhibitors available for Fas receptor-associated proteins which are components of the death-induced signaling complex.
With the recent advent of RNA interference (RNAi) technology, the blockade of certain genes involved in pathologic mechanisms is being carried out extensively in vitro, but this has rarely been tried in vivo because of the inefficiency of various vectors and their potential inflammatory sequelae. However, using a technique known as hydrodynamic delivery, naked constructs, including double-stranded small interfering RNAs (siRNAs) can be introduced into the tissues of the whole animal by increasing its hydrostatic pressure with a rapid large volume tail-vein injection.7 Recently, a model of concanavalin A/Fas-induced fulminant hepatitis has shown that siRNA targeted against Fas can effectively reduce Fas message when given hydrodynamically (siRNA dissolved in 2 mL volume, injected via the tail-vein over 5 seconds) and can maintain a decreased level of the message for up to 10 days.8 Similarly, siRNA targeted against caspase-8, a downstream signaling molecule in the Fas pathway, has also been used in a FasL-adenovirus mouse model of liver damage and shown to maintain decreased caspase-8 mRNA and improve survival.9 However, although these observations are informative, these were largely proof-of-principle experiments in which the mediator/causative mechanism was established, unlike sepsis.
Because the hydrodynamic administration of siRNA is reported to have a prolonged, but not permanent, effect on its target mRNA, we used this technique in the model of polymicrobial sepsis CLP to determine whether the blockade of Fas or caspase-8 attenuates the onset of morbidity and mortality seen in polymicrobial sepsis.
Materials and methods
Animals
The initial gene-silencing and tissue-distribution studies with siRNA were carried out using female C57BL/6-TgN (ACTbEGFP)/Osb (green fluorescent protein [GFP] transgenic)10 mice (Jackson Laboratories, Bar Harbor, ME). Subsequently, male C3H/HeN (TLR4 deficient; Charles River Laboratories, Wilmington, MA), 6 to 8 weeks of age were used in Fas and caspase-8 siRNA experiments. The studies described here were carried out in accordance with the National Institutes of Health guidelines and the National Research Council's Institute of Laboratory Animal Resources (ILAR) Guide for the Care and Use of Laboratory Animals, and were approved by the Brown University and Rhode Island Hospital Institutional Animal Care and Use Committee.
Model of sepsis
The surgical procedure to generate sepsis was carried out as previously described.11 C3H/HeN male mice were lightly anesthetized using isoflorane (Abbott Laboratories, North Chicago, IL). The abdomen was shaved and scrubbed with povidone-iodine. A midline incision (1.5-2 cm) was made below the diaphragm. The cecum was isolated, ligated, punctured twice with a 22-gauge needle, and was gently compressed to extrude a small amount of cecal material. The cecum was returned to the abdomen, and the muscle and skin incisions were closed with 6-0 Ethilon suture material (ETHICON, Somerville, NJ). Before suturing the skin, 2 to 3 drops of lidocaine (Abbott Laboratories) was administered to the wound for analgesia. The mice were subsequently resuscitated with 1.0 mL lactated Ringer solution subcutaneously. Sham controls were subjected to the same surgical laparotomy and cecal isolation, but the cecum was neither ligated nor punctured.
Delivery of siRNA
Fas, caspase-8, and GFP siRNA were obtained from Dharmacon RNA Technologies (Lafayette, CO). The target sequence used for Fas was 5′-AAGUGCAAGUGCAAACCAGAC-3′8 ; for caspase-8, it was 5′-AACCUCGGGAUACUGUCUGA-3′.9 The GFP siRNA target sequence was, as produced by Dharmacon, 5′-GGCTACGTCCAGGAGCGCACC-3′. For tissue distribution studies, GFP mice were injected with either 2 mL saline or 50 μg GFP siRNA dissolved in 2 mL saline. Twenty-four hours later the mice were killed, and organs were harvested for frozen tissue sectioning in optimum cutting temperature (OCT) medium. Frozen tissues were sectioned on a cryostat (Leica, Bannockburn, IL) and visualized using a Nikon Eclipse E400 fluorescent microscope (Nikon, Melville, NY) to assess change in GFP intensity after administration of GFP siRNA. Images were acquired with a Polaroid digital microscope camera and software (Polaroid, Waltham, MA). The qualitative change in GFP intensity and the overall number of fluorescing cells per field was made by an investigator blinded to the sample identity. The scores were +++, highly fluorescent (50%-100% of cells in field fluorescent); ++, moderately fluorescent (25%-49%); +, weakly fluorescent (24%-5%); -, background/nonfluorescent (4%-0%).
For the majority of the in vivo Fas and caspase-8 assessment studies, C3H/HeN mice were randomly placed into groups receiving 50 μg naked Fas, caspase-8, or GFP siRNA/mouse or 1.5 mL normal saline alone in the form of a hydrodynamic intravenous injection 30 minutes after CLP. However, in some studies the administration of siRNA was delayed up to 12 hours after CLP. Typically, 50 μg siRNA was dissolved in 1.5 mL phosphate-buffered saline (PBS) and was then injected rapidly (over a period of 5 seconds) into the tail vein. In these studies GFP siRNA was used as a nonsense siRNA control.8 It should be noted that a mouse genome BLAST12 search indicated the sequence interacted with no known sequence in the mouse.
Assessment of IFN-α and IL-6 activation
C3H/HeN mice were injected with either 200 μg poly I:C (deoxyinosine-deoxycytosine) (Sigma, Saint Louis, MO) to achieve a positive signal, 50 μg caspase-8, or Fas siRNA, or were left untreated. The plasma was harvested 18 hours after injection to analyze circulating levels of interferon α (IFN-α) or interleukin 6 (IL-6) via a sandwich method enzyme-linked immunosorbent assay (ELISA) kit (Antigenix America, Huntington Station, NY, or BD Pharmingen, San Diego, CA, respectively) and was carried out according to manufacturer's instructions (n = 4/group).
Reverse transcriptase (RT)-PCR
For Fas and caspase-8 mRNA analysis, RNA was isolated from livers of sham or CLP mice treated with saline, GFP, Fas, or caspase-8 siRNA. Reverse transcription using an iScript kit (Bio-Rad Laboratories, Hercules, CA) was performed to generate cDNA. Polymerase chain reaction (PCR) was carried out for 30 cycles using 2.5 μL cDNA and 22.5 μL PCR Master Mix containing Taq DNA polymerase (Promega, Madison, WI). Custom primers were used and are as follows: Fas, AAAGTGGCCCATTTAACAGGC (forward) and AAAGCAGGAC AATTCCATAGGTG (reverse); caspase-8, TGCCCTCAAGTTCCTGTGCTTGGAC (forward) and GGATGCTAAGAATGTCATCTCC (reverse); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TGCATCCTGCACCACCAACT (forward) and AACACGGAAGGCCATGCCAG (reverse). Densitometric analysis was done with an Alpha-Innotek gel documentation station (Alpha-Innotek, San Leandro, CA).
Western blot
Protein lysates of mouse liver and spleen were run on 12% Tris (tris[hydroxymethyl]aminomethane)-glycine gels (Invitrogen, Carlsbad, CA). Blotting procedures, chemiluminescent detection, and densitometric analysis were carried out as previously described by our laboratory.13 Membranes were probed with Fas rabbit polyclonal antibody, caspase-8 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), active caspase-3 (BD Pharmingen), or pro-caspase-9 (Chemicon, Temecula, CA).
Assessment of apoptosis
Twenty-four hours after CLP, mice that had been treated with Fas siRNA, caspase-8 siRNA, GFP siRNA (nonsense control),8 or saline were killed to harvest liver and spleen. Both tissues were processed for frozen sectioning. Frozen section slides were fixed with 4% paraformaldehyde and washed with PBS. Slides were incubated with transferase-mediated dUTP (deoxyuridine triphosphate) nick end labeling (TUNEL) reaction mixture according to manufacturer's protocol (Roche Diagnostics, Indianapolis, IN). Degree of apoptosis was assessed by visualizing tissues stained with 4,6 diamidino-2-phenylindole (DAPI) using a Nikon Eclipse E400 fluorescent microscope. Apoptotic index was derived from the average of the number of TUNEL-positive cells versus the total number of cells in 8 random fields at 600 × magnification (visualized with a 60 ×/0.85 objective lens). Images were acquired with a Polaroid DMC2 digital microscope camera and DMC2 2.0.1 software.
Caspase-3 and caspase-8 activity assays
As described previously by Chung et al,14 livers and splenocytes from septic mice after being treated with saline, GFP, Fas, or caspase-8 siRNA were homogenized in the presence of lysis buffer containing dithiothreitol (DTT). Two times reaction buffer containing DTT and AFC-DEVD (caspase-3) or AFC-IETD (caspase-8) (BioVision, Mountain View, CA) was added to 50 μg splenocyte lysate or 400 μg liver lysate. After 1 hour of incubation, samples were read on a fluorescent plate reader (FLx800; Bio-Tek, Winoski, VT) at excitation 400 nm and emission 505 nm to determine the extent of AFC release in arbitrary fluorescent units.
Plasma liver enzyme levels
After being treated with saline, GFP, Fas, or caspase-8 siRNA blood from septic mice was collected in a syringe containing 2 U heparin, transferred to a microtube, and centrifuged at 10 000g for 10 minutes at 4°C. Plasma samples were stored at -80°C until assayed. Plasma AST and ALT levels were determined using a kit (Biotron Diagnostics, Hemet, CA), according to the manufacturer's instructions.
Survival studies
Approximately 30 minutes following CLP, mice were placed randomly in groups (20-22 mice/group), receiving either saline, GFP siRNA, Fas siRNA, or caspase-8 siRNA in the form of a hydrodynamic injection. Mice were returned to the animal facility, and the percentage of survival was followed and recorded for 10 days.
Statistics
The data are presented as a mean and SE of the mean for each group. Differences in percentile (ie, apoptotic index percentage) data were considered to be significant at a P value of less than .05, as determined by the Mann-Whitney rank sum test. Changes in ALT and AST levels and IFN-α levels were considered significant at a P value of less than .05, as determined by the unpaired t test. Results of the survival studies were compared using the chi-square test and were considered significant at a P value of less than .05.
Results
Tissue distribution of siRNA after hydrodynamic delivery
Because in vivo use of siRNA has been applied only in mediator-defined models specific to the liver,8,9 we sought to initially establish the capacity of siRNA to suppress specific GFP transgene expression and to determine the tissue distribution of siRNA when injected hydrodynamically. To investigate this, GFP transgenic mice were injected hydrodynamically with 50 μg GFP siRNA or a saline vehicle. Tissues were then examined using fluorescence microscopy to assess the extent of GFP suppression as manifested by a decrease in fluorescence in the siRNA-treated animal. We observed that in all the GFP mouse tissues examined, including spleen and liver (Figure 1) as well as heart, kidney, lung, muscle, brain, thymus, and Peyer patches (data not shown), a decrease in GFP was evident. This indicates that hydrodynamic administration suppresses, but does not totally ablate, GFP gene expression, while also providing an effective distribution of siRNA to all major organs and cells.
Systemic delivery of siRNA does not activate IFN-α and IL-6
C3H/HeN mice injected with siRNA against caspase-8 or Fas siRNA alone were analyzed for an increase in interferon activation as typically seen in response to double-stranded RNA (dsRNA). Levels of IFN-α in the plasma of mice treated with siRNA (67 ± 8 pg/mL) were similar to levels in naive, untreated mice (33.5 ± 1.5 pg/mL), as compared (P < .05) with the increased levels seen in mice treated with poly I:C (233 ± 50 pg/mL). IL-6 blood levels exhibited a change comparable to that of IFN-α, with levels of about 83.7 ± 6 pg/mL for naive animals and 49.2 ± 9 pg/mL for siRNA-treated animals versus a rise of 3166 ± 11.2 pg/mL in mice treated with poly I:C (n = 4 for each group).
Tissue distribution and the ability of hydrodynamically delivered GFP siRNA to silence GFP expression in GFP-transgenic mice, seen at 600 × magnification. Suppression of green fluorescent protein was seen in the liver, spleen, thymus, lung, muscle, heart, kidney (shown), brain, and Peyer patches (not shown). The qualitative change in GFP intensity and the overall number of fluorescing cells per field was made by an investigator blinded to the sample identity.
Tissue distribution and the ability of hydrodynamically delivered GFP siRNA to silence GFP expression in GFP-transgenic mice, seen at 600 × magnification. Suppression of green fluorescent protein was seen in the liver, spleen, thymus, lung, muscle, heart, kidney (shown), brain, and Peyer patches (not shown). The qualitative change in GFP intensity and the overall number of fluorescing cells per field was made by an investigator blinded to the sample identity.
Hydrodynamic delivery of Fas or caspase-8 siRNA suppresses Fas or caspase-8 mRNA and protein expression, respectively, in septic mice
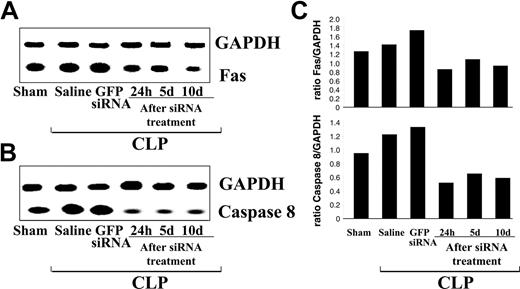
Because Fas signaling is up-regulated in sepsis, RT-PCR was used to evaluate the mRNA expression levels of both Fas and caspase-8 after siRNA treatment after CLP versus separate control sets for gene targeting (Figure 2A-B). As compared with saline- and GFP siRNA-treated controls, mice treated with caspase-8 or Fas siRNA show an approximate 40% reduction in the level of caspase-8 or Fas mRNA in the liver, respectively, comparable to the levels found in the sham-CLP mouse (Figure 2A-B). This suppression seems to be sustained up to day 10 after CLP, as the hepatic caspase-8 or Fas expression was still only about 65% of the sham-CLP group.
Semiquantitative RT-PCR showing a representative change mRNA levels of caspase-8 and Fas in the liver relative to GAPDH after siRNA treatment. Total RNA was isolated from mice 24 hours, 5 days (d5), or 10 days (d10) after CLP or sham surgery. Animals that underwent CLP received normal saline, GFP siRNA, Fas siRNA, or caspase-8 siRNA 30 minutes after surgery. (A) Fas mRNA levels after CLP/Fas siRNA treatment. (B) caspase-8 mRNA levels after CLP/caspase siRNA treatment. Levels are decreased to sham levels at 24 hours, 5 days, and 10 days after CLP/Fas or caspase-8 siRNA treatment. (C) Densitometry analysis of the intensity of mRNA bands for Fas and caspase-8 relative to GAPDH. Experiment was repeated three times.
Semiquantitative RT-PCR showing a representative change mRNA levels of caspase-8 and Fas in the liver relative to GAPDH after siRNA treatment. Total RNA was isolated from mice 24 hours, 5 days (d5), or 10 days (d10) after CLP or sham surgery. Animals that underwent CLP received normal saline, GFP siRNA, Fas siRNA, or caspase-8 siRNA 30 minutes after surgery. (A) Fas mRNA levels after CLP/Fas siRNA treatment. (B) caspase-8 mRNA levels after CLP/caspase siRNA treatment. Levels are decreased to sham levels at 24 hours, 5 days, and 10 days after CLP/Fas or caspase-8 siRNA treatment. (C) Densitometry analysis of the intensity of mRNA bands for Fas and caspase-8 relative to GAPDH. Experiment was repeated three times.
In addition, we found that both caspase-8 and Fas siRNA were able to suppress protein levels of caspase-8 or Fas in the liver or spleen through day 10 after injection (Figure 3A-B). Densitometry analysis showed that the hydrodynamic injection of siRNA suppressed its target mRNA in the liver by approximately 40% (Figure 2C) and that specific protein (Figure 3B,D) expression was alternatively reduced by at least 40% to 60%.
Fas or caspase-8 siRNA suppresses apoptosis after sepsis in the spleen and liver
Because numerous studies have previously documented dysregulated apoptosis of the immune system, particularly lymphocytes15 and to a lesser extent myeloid cells4,16 and nonparenchymal cells, we set out to determine the effect of Fas or caspase-8 treatment on the onset of septic apoptosis. Figure 4A illustrates what was typically found following CLP: an increase in the incidence of TUNEL-positive cells observed in the CLP mouse liver (Figure 4Ai) and spleen (not shown) which was not affected by treatment with GFP siRNA (Figure 4Aii). The number of TUNEL-positive cells declined in Fas siRNA-treated CLP mice (Figure 4Aiii). Quantitation of these changes (Figure 4B) shows that Fas and caspase-8 siRNA given after CLP decrease the apoptotic index in the spleen and the liver to sham levels. siRNA treatment did not, however, have an effect on apoptosis in the thymus (Figure 4C). Figure 5A shows active caspase-3 levels in the liver and the spleen, confirming the TUNEL findings that Fas or caspase-8 siRNA treatment after CLP decreases apoptosis. In addition, increased levels of pro-caspase-9 in the liver (Figure 5B) and spleen (not shown) after Fas or caspase-8 siRNA treatment indicate a reduction in apoptosis in these organs. Last, caspase-3 and -8 activity is decreased with Fas or caspase-8 treatment (Figure 5C).
Protein levels in both the liver and spleen after Fas or caspase-8 treatment as shown by Western blot. Total protein was isolated from mice 24 hours, 5 days (d5), or 10 days (d10) after CLP or sham surgery. Western blot band density (in arbitrary densitometric units, AU) for Fas (A, B) and caspase-8 (C, D). Blots in panels A and C are representative of 3 separate repeat experiments. ▪ represents the liver; □, the spleen. *P < .05 versus CLP/saline and CLP/GFP siRNA controls, t test (n = 4/group). Data are presented as mean ± SE of the mean.
Protein levels in both the liver and spleen after Fas or caspase-8 treatment as shown by Western blot. Total protein was isolated from mice 24 hours, 5 days (d5), or 10 days (d10) after CLP or sham surgery. Western blot band density (in arbitrary densitometric units, AU) for Fas (A, B) and caspase-8 (C, D). Blots in panels A and C are representative of 3 separate repeat experiments. ▪ represents the liver; □, the spleen. *P < .05 versus CLP/saline and CLP/GFP siRNA controls, t test (n = 4/group). Data are presented as mean ± SE of the mean.
Apoptosis in the liver and spleen following siRNA treatment. (A) Representative immunofluorescent micrographs indicating TUNEL-positive cells in liver sections after CLP; (i) saline treatment, (ii) GFP siRNA, and (iii) Fas siRNA. (B) Apoptotic index of hepatocytes and splenocytes following caspase-8 and Fas siRNA treatment following CLP as determined by TUNEL. ▪ indicates hepatocytes; □, splenocytes. *#P < .005 versus saline and GFP controls, Mann-Whitney U test (n = 5/group). (C) Apoptotic index in the thymus following caspase-8 or Fas siRNA treatment following CLP. Data are presented as mean ± standard error (SE) of the mean.
Apoptosis in the liver and spleen following siRNA treatment. (A) Representative immunofluorescent micrographs indicating TUNEL-positive cells in liver sections after CLP; (i) saline treatment, (ii) GFP siRNA, and (iii) Fas siRNA. (B) Apoptotic index of hepatocytes and splenocytes following caspase-8 and Fas siRNA treatment following CLP as determined by TUNEL. ▪ indicates hepatocytes; □, splenocytes. *#P < .005 versus saline and GFP controls, Mann-Whitney U test (n = 5/group). (C) Apoptotic index in the thymus following caspase-8 or Fas siRNA treatment following CLP. Data are presented as mean ± standard error (SE) of the mean.
Fas and caspase-8 siRNA treatment after CLP decreases plasma liver enzymes
Earlier work has already demonstrated that blocking Fas decreases the extent of liver damage after sepsis.4,5 Hepatocellular damage has been reported to occur early during experimental sepsis and is evidenced by an elevation in plasma ALT and AST levels.17,18 In this respect, we observed that when mice were treated with either Fas or caspase-8 siRNA, but not GFP siRNA, there was a significant (n = 6/group; P < .05) attenuation of liver enzyme ALT and AST levels of about 30% from the peak levels (28 ± 3 ALT units/L and 39 ± 2 AST units/L/group) seen in the CLP/saline vehicle group (data not shown).
Levels of active caspase-3 in the liver and the spleen. These blots are representative of 3 repeat experiments. (A) Active caspase-3 and (B) procaspase-9 levels in the liver after siRNA treatment. (C) Caspase-3 (left graph) and 8 (right graph) activities (in arbitrary fluorescent units) in the liver and the spleen after CLP and Fas or caspase-8 siRNA treatment. Data are presented as mean ± SE of the mean.
Levels of active caspase-3 in the liver and the spleen. These blots are representative of 3 repeat experiments. (A) Active caspase-3 and (B) procaspase-9 levels in the liver after siRNA treatment. (C) Caspase-3 (left graph) and 8 (right graph) activities (in arbitrary fluorescent units) in the liver and the spleen after CLP and Fas or caspase-8 siRNA treatment. Data are presented as mean ± SE of the mean.
Treatment with Fas and caspase-8 siRNA improves survival of mice with polymicrobial sepsis
To the extent that hydrodynamic delivery of siRNAs targeting Fas or other downstream signaling molecules in the pathway (caspase-8) could provide a survival advantage to septic mice, we initially followed septic mortality of mice receiving siRNA, approximately 30 minutes following the performance of CLP, for up to 12 days. In this study, mice injected with caspase-8 or Fas siRNA after septic insult exhibit a significant increase in survival at 3 days after CLP and beyond when compared with saline or GFP siRNA controls (Figure 6A-B).
Delayed administration of siRNA against Fas still provides a survival benefit to polymicrobial septic mice
Figure 6C, inset, illustrates the survival of mice that received a 2-mL hydrodynamic injection of normal saline 12 hours after CLP as compared with mice that received only the normal 0.8 mL lactated Ringer solution immediately after CLP. Septic mice receiving an injection 12 hours after CLP show a transient improvement of survival at 36 hours after CLP, suggesting that the extra fluids provide some support during the immune hyporesponsive phase of sepsis and also that the large fluid volume injection is not itself deleterious to the septic mouse. Interestingly, when Fas siRNA suspended in normal saline was included in the resuscitation, given at 12 hours after CLP, a significant survival benefit was still evident (Figure 6C) in these animals when compared with vehicle-treated mice.
Discussion
Our studies have demonstrated that gene transcripts necessary for apoptosis in sepsis can be silenced or suppressed in the septic animal via RNAi. To accomplish this silencing in vivo, siRNA targeting genes of interest can be introduced into the whole animal and reach major organs through a rapid large volume injection. With this in mind, we report that siRNA targeting our genes of interest, Fas and caspase-8, could effectively be delivered to target organs, those of which are already known to have increased incidence of Fas-driven apoptosis upon septic insult.4 Because siRNA was able to decrease Fas and caspase-8 mRNA and protein levels, it was not surprising that this treatment was also able to decrease apoptosis in the spleen and liver. Although we observed evidence of gene silencing in the thymus, based on the reduction of GFP signal in the GFP transgenic mice with GFP siRNA, septic thymic apoptosis was not decreased. This, however, is not surprising because we19 and other laboratories20 have shown that the thymus does not seem to undergo Fas-mediated apoptosis, but undergoes steroid-induced apoptosis. To this extent, the decrease in apoptosis prevents injury to the liver. We also noted that treatment with either Fas or caspase-8 siRNA also decreased plasma liver enzymes, ALT and AST.
Survival of septic mice after siRNA treatment. (A) Survival of mice receiving saline, GFP siRNA, or caspase-8 siRNA 30 minutes after CLP (time of injection indicated by red arrow). Significant survival protection is seen at day 3. *P < .05 versus saline and GFP controls, chi-square test (n = 20/group). (B) Survival of mice receiving saline, GFP siRNA, or Fas siRNA 30 minutes after CLP. Significance in survival is also seen at day 3. *P < .05, chi-square test (n = 22 /group). (Ci) Percentage of survival of mice receiving a hydrodynamic injection (2 mL) of normal saline 12 hours after CLP, as compared with normal resuscitation, 0.8 mL lactated Ringer solution immediately following CLP. (Cii) The effect of Fas siRNA given hydrodynamically 12 hours after CLP (injection time indicated by arrow). A significant survival benefit is seen at day 5, *P < .05 versus saline and GFP controls, chi-square test (n = 15-17/group).
Survival of septic mice after siRNA treatment. (A) Survival of mice receiving saline, GFP siRNA, or caspase-8 siRNA 30 minutes after CLP (time of injection indicated by red arrow). Significant survival protection is seen at day 3. *P < .05 versus saline and GFP controls, chi-square test (n = 20/group). (B) Survival of mice receiving saline, GFP siRNA, or Fas siRNA 30 minutes after CLP. Significance in survival is also seen at day 3. *P < .05, chi-square test (n = 22 /group). (Ci) Percentage of survival of mice receiving a hydrodynamic injection (2 mL) of normal saline 12 hours after CLP, as compared with normal resuscitation, 0.8 mL lactated Ringer solution immediately following CLP. (Cii) The effect of Fas siRNA given hydrodynamically 12 hours after CLP (injection time indicated by arrow). A significant survival benefit is seen at day 5, *P < .05 versus saline and GFP controls, chi-square test (n = 15-17/group).
From an outcome perspective, we observed that, importantly, suppression of Fas or caspase-8 gene expression with this in vivo siRNA administration also conferred a survival advantage to septic mice as compared with controls. Interestingly, this survival benefit was still evident even when the administration of Fas siRNA was provided as late as 12 hours after CLP. This would suggest that Fas-mediated pathologic effects are sustained over time or require a longer period of time to develop. These findings are also in keeping with the concept that the suppression of genes in this fashion can last 5 to 10 days, based on the reports of others, as well as that seen here.8 That said, although we found that the suppression of both message and protein production in the liver lasts up to 10 days in our model, we have not explored the nature of this persistence. At present, we can only speculate that under in vivo conditions, cells in the liver (which do not divide like cell lines in culture, to which comparisons are made), lead to less dilution of the functional siRNA levels present and thus may allow the silencing effect to persist much longer.8 This also suggests that protection from Fas-activation-induced pathology might be comparable at 24 hours after CLP whether the siRNA was given at 30 minutes or 12 hours after CLP. This, however, remains to be established in this model.
These results, together with our observation that the loss of caspase-9 proform and the increase in caspase-3 and -8 activity in septic mice can be suppressed, further support the role of the extrinsic/Fas-mediated cell-death pathway as well as the intrinsic/mitochondrial apoptotic process in the pathology of sepsis. Our findings also demonstrate the potential to target downstream enzymatic as well as nonenzymatic components of this pathway as possible therapeutic targets. The point being here that as more is understood about the mechanism of RNAi action, its delivery and uptake of siRNA into cells, this method could be modified for use in the clinical setting. Another possible approach is the use of cationic liposomes encoding siRNAs. This technique was used by Sorensen et al21 as a pretreatment to endotoxemia. Liposome-based anti-TNF-α siRNAs given intraperitoneally 18 hours prior to LPS injection significantly improved survival of BALB/C mice and decreased TNF-α levels. That said, many gene-based treatments necessitate the use of vectors, many of which cause inflammation and other side effects that minimize their use in inflammatory conditions such as those experienced by the septic animal.22,23 Thus, it was a concern that the in vivo infusion of siRNA might induce an inflammatory response through the activation of the interferon pathway.20 However, we have found that animals treated solely with siRNA did not show an increase in plasma IFN-α levels, which have been well-documented as rising in response to TLR3, RNA-dependent protein kinase, and/or IFN regulatory factor-signal transducer and activator of transcription-1 activation by double-stranded RNA,24,25 whereas the administration of poly I:C did induce such a change. This would suggest that hydrodynamic delivery of siRNA at this concentration differs distinctly from alternative forms of double- or single-stranded RNAs as a stimulant of interferon activation.
By using hydrodynamic delivery of plasmid DNA, it has previously been shown that volume and rate of injection are critical in the uptake of naked constructs, in that it must be a large fluid volume (ie, equivalent of about one time the animal's blood volume) rapidly injected.7 Liu et al7 have shown that the injected transgene can be expressed in the lung, spleen, heart, kidney, and more extensively in the liver. Our findings are consistent with Liu et al7 ; even though the construct we used was naked siRNA, we were still able to observe its silencing ability in all major organs after hydrodynamic delivery.
Because of the nature of hydrodynamic injection, there are obvious clinical limitations with respect to volume and rate given in this way. However, our main objective here was to document that the silencing of upstream and downstream gene targets of an important proapoptotic pathway could be achieved by using a posttreatment siRNA approach in sepsis, so the nature and mode of delivery was not our primary concern. Nonetheless, we do appreciate that there are attributes of fluid resuscitation in septic patients that need to be considered.26,27 Here, we explored the effects of hydrodynamic delivery in septic mice and whether a large volume injection of normal saline would cause any untoward effect on survival from greater resuscitation volume. We found, however, that hydrodynamic fluid delivery had no marked deleterious effects when administered at either 30 minutes, 1 hour, or 12 hours after CLP. If anything, there was a transient survival benefit at 36 hours after CLP which was subsequently lost. It is also important to appreciate that all arms of this study received hydrodynamic fluid administration. Thus, the differences seen would be independent of those effects attributable simply to administration of a large volume of fluid alone.
The question remains, however, as to what cells are targeted by Fas or caspase-8 siRNA treatment in this model of sepsis that are enabling the animal to survive. Increased Fas expression is seen on leukocytes, particularly neutrophils and monocytes, of humans treated with endotoxin.28 After endotoxin treatment, and subsequent systemic inflammation, Fas mRNA expression is increased, indicating de novo transcription and increased surface expression of Fas.28 This, in turn, increases the susceptibility of target cells to Fas-mediated apoptosis. Not surprisingly, leukocytes of septic patients also exhibit an increase in Fas receptor expression, whereby higher Fas expression correlates with a higher rate of mortality.29 A similar phenomenon for FasL has also been recently observed in the spleen of bacteremic baboon.26 This is in keeping with the idea that the immune hyporesponsive stage of sepsis contributes to the animal's inability to defend itself against the lethal polymicrobial challenge.30 With respect to Fas ligand, there is an increase in the liver after experimental sepsis in mice, but not in the spleen,31 suggesting that it is the increase in Fas death receptor that is responsible for the increase in apoptosis in these organs. In this respect, our laboratory has also made a similar finding of increased splenic lymphoid Fas but not FasL gene expression in septic mice.32 Lymphocytes are also important modulators of the immune response to sepsis. In experimental animals, we see these cells become immunocompromised at the switch from the hyperresponsive to the hyporesponsive stage of sepsis (> 12 hours after CLP).30 In patients, many clinically based studies have correlated increased apoptosis, particularly in lymphocytes, with infection, organ dysfunction, and decreased survival of sepsis,33-35 possibly resulting from this immunocompromised state. It has been suggested in mouse models that blocking apoptosis of immune cells improves survival35 and can provide a benefit to the nonparenchymal cells of an organ such as the liver, because it would prevent bystander cellular damage.4 In this model, liver, spleen, and thymic-cell apoptosis was analyzed by TUNEL and active caspase-3 expression after treatment with Fas or caspase-8 siRNA. We see a significant decrease in apoptosis by the decline in TUNEL-positive cells, as well as a decrease in active caspase-3 levels in tissues that usually undergo Fas-mediated apoptosis in sepsis, such as the liver and the spleen. It is possible that this is a “rescue” that is maintaining immune competency in the mouse, thus preventing the bystander cellular damage, multiple-organ failure, and subsequent morbidity that are normally seen in sepsis. The studies presented here use a simple way of introducing siRNA directly into the animal without the use of a vector. This is an efficacious method for silencing or suppressing genes for apoptosis after transcription that are normally up-regulated in the septic state, and for that reason are found to contribute to septic mortality from multiple-organ failure. Intriguingly, the capacity to alter the septic response in this fashion implies that various downstream components of not only apoptotic but also nonapoptotic pathways may also be potential targets for this type of siRNA approach. The use of Fas and caspase-8 siRNA not only identifies these as potential upstream and downstream therapeutic targets in sepsis but also confirms the role of the Fas/FasL signaling pathway in the septic syndrome.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2004-10-4086.
Supported by the National Institutes of Health (grant R01-GM53 209) (A.A.) and Graduate Assistance in Areas of National Need (GAANN; training grant P200A030100) (D.E.W.-S.).
Presented in part at the 27th annual conference of Shock's New Investigator Competition, Halifax, NS, Canada, June 6, 2004.36
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Yaping Chen, Ms Tina Rachel, and Ms Maryann San Martin for their technical assistance. We also thank Ms Colleen Bandarra for her assistance in preparation of this manuscript.