Abstract
The long-term efficacy and toxicity of hydroxyurea for infants are undefined, and its role in preventing organ dysfunction is unknown. Short-term feasibility of hydroxyurea administration, toxicities, hematologic effects, and effect on spleen function in infants with sickle cell anemia (SCA) were reported (Hydroxyurea Safety and Organ Toxicity [HUSOFT] trial). These infants completing 2 years of hydroxyurea therapy (20 mg/kg/d) were offered study extension with dose escalation to 30 mg/kg/d. Patients were monitored with laboratory tests and biannual imaging studies. Hematologic indices were compared with predicted age-specific values and event rates compared with historic rates. All 21 subjects completing the original trial enrolled in the extension study: median age, 3.4 years old (range, 2.6 to 4.4 years); 12 females; 20 with Hb SS, 1 with Hb S/β0-thalassemia. Seventeen patients completed 4 years of hydroxyurea, and 11 completed 6 years. After 4 years, hydroxyurea was associated with increased hemoglobin concentration, percentage of fetal hemoglobin (Hb F), and mean corpuscular volume (MCV) and decreased reticulocytes, white blood cells (WBCs), and platelets (P < .01). Patients experienced 7.5 acute chest syndrome (ACS) events per 100 person-years, compared with 24.5 events per 100 person-years among historic controls (P = .001). Treated patients had better spleen function than expected and improved growth rates. Infants with SCA tolerate prolonged hydroxyurea therapy with sustained hematologic benefits, fewer ACS events, improved growth, and possibly preserved organ function.
Introduction
The clinical manifestations of sickle cell anemia (SCA) result primarily from hemolytic anemia and the effects of repeated intravascular sickling, causing vasoocclusion and ischemic injury. Chronic organ damage in SCA is an insidious process that may affect almost every organ system and can lead to considerable morbidity and mortality at an early age. Loss of splenic function,1 sickle nephropathy (proteinuria and renal insufficiency),2 pulmonary hypertension,3 and brain ischemic lesions4 are examples of long-term end-organ damage observed in SCA. Lower levels of fetal hemoglobin (Hb F) and higher white blood cell (WBC) counts are associated with an increased incidence of SCA-related events, organ damage, and mortality.5
Hydroxyurea is an antimetabolite chemotherapic agent known to stimulate fetal hemoglobin production,6,7 thereby offering a therapeutic option for the prevention of acute vasoocclusive events (VOEs) in SCA. Over the past decade, several trials have demonstrated that hydroxyurea is an efficacious therapy for both adult and pediatric patients with SCA.8-13 In the pediatric age group, however, there have been very few controlled studies of hydroxyurea therapy, so neither efficacy nor effectiveness has been clearly defined in this patient population.
Hydroxyurea has the ability to raise Hb concentration and Hb F values while increasing red-cell mean corpuscular volume (MCV).9,11,14,15 Hydroxyurea has additional effects, including lowering the WBC, reticulocyte, and platelet counts; increasing nitric oxide production16 ; decreasing red blood cell intracellular dehydration17 ; and decreasing red-cell adhesiveness to endothelium.18 Clinically, hydroxyurea has been shown to decrease the rate of pain and acute chest syndrome (ACS) events in severely affected adults and children.8-11,19,20
Organ damage starts very early in life, and most children with SCA lose splenic reticuloendothelial function by 2 years of age.1 The efficacy of hydroxyurea for the prevention of chronic organ damage in SCA and the potential benefit of dose escalation have not been fully investigated. Recovery of splenic function during hydroxyurea therapy has been reported anecdotally,15,21,22 but prospective investigation has been limited.
The Hydroxyurea Safety and Organ Toxicity (HUSOFT) trial was a prospective, multicenter, open-label, single-arm, pilot study designed to (1) examine the feasibility of liquid hydroxyurea administration in infants with SCA, (2) determine the toxicities of hydroxyurea in this very young age group, (3) assess the effects of hydroxyurea on fetal Hb levels and other hematologic indices, and (4) obtain pilot data regarding the potential of hydroxyurea to preserve splenic function.23 In the previously published 2-year pilot study, 28 infants with SCA, all unselected for disease severity, were prospectively treated. Hydroxyurea was well tolerated, produced mild toxicities (predominantly transient neutropenia), maintained elevated Hb concentration and percent Hb F levels, and possibly prevented loss of spleen function.23 All 21 infants completing the original 2-year pilot study were offered continued liquid hydroxyurea therapy. This extension of the original HUSOFT trial investigated the effect of hydroxyurea dose escalation on hematologic response and provides long-term prospective data regarding the safety and efficacy of hydroxyurea therapy in this cohort of very young children with SCA.
Patients, materials, and methods
Patient selection and treatment
The original HUSOFT trial was designed to offer hydroxyurea to infants aged 6 to 24 months with the diagnosis of homozygous sickle cell anemia (Hb SS) or Hb Sβ0-thalassemia regardless of disease severity.23 Patients were enrolled at 4 major pediatric sickle cell programs from November 1996 through June 1997. Using a common protocol approved by each local institutional review board (IRB), hydroxyurea was given orally once a day at the fixed dose of 20 mg/kg administered as a flavored liquid formulation prepared by dissolving the contents of hydroxyurea capsules at a final concentration of 100 mg/mL. The chemical and functional stability of this liquid formulation has been reported recently.24
Twenty-one infants completed 2 years of hydroxyurea treatment in the original HUSOFT trial, and each family was offered continuation of hydroxyurea therapy in the HUSOFT extension. All 21 patients were enrolled, and their hydroxyurea dose was escalated by 5 mg/kg every 6 months to a maximum dose of 30 mg/kg/d. Parents were instructed by several members of the health care team on how to take the medication, were given calendars to help remind them, and were asked to return their medication bottles to help assess compliance. Institutional review board approval and informed consent were obtained for continued hydroxyurea treatment, imaging, and laboratory evaluation, in accordance with the Declaration of Helsinki.
Patient evaluation, monitoring, and toxicity
Patients were followed with biweekly physical examinations, including weight, height, and head circumference measurements, and blood counts for the first 2 months after dose escalation and then monthly thereafter. Hb F and F-cell measurements were performed every 6 months as previously described.25 Alanine amino transferase (ALT) and creatinine were measured quarterly. Splenic sequestration and painful events requiring hospitalization were documented at each visit. Splenic sequestration was defined by a decline from baseline of Hb concentration of 2 g/dL (20 g/L) or more, an increase in reticulocyte count, and an acutely enlarged spleen. ACS was defined as a new infiltrate on the chest x-ray accompanied by respiratory symptoms and/or fever. Pain episodes were defined as pain in the extremities, back, abdomen, chest, or head for which no other explanation could be found.
Spleen filtrative function was ascertained through technetium 99m (Tc99m) sulfur-colloid uptake measurement at baseline and after 2 and 4 years of therapy. A single nuclear medicine radiologist, blinded to age of the subjects and prior liver-spleen scan results, interpreted the films. Splenic uptake was qualitatively evaluated as normal, decreased, markedly decreased, or no uptake, as previously described.26 At the institutions where magnetic resonance imaging (MRI) and angiography (MRA) of the brain were readily available, testing was performed at 2-year intervals and evaluated by local neuroradiologists who were unaware of the patients'clinical status.
Hematologic toxicity was defined as an absolute neutrophil count (ANC) less than 1.5 × 109/L, platelet count less than 80 × 109/L, decline in Hb concentration more than 20% from baseline, or a Hb concentration less than 5.0 g/dL (50 g/L). Renal and hepatic toxicity were defined as an increase in serum creatinine by more than 50% or ALT greater than twice the upper limit of normal. If toxicity was identified, hydroxyurea was temporarily discontinued. Patients were reevaluated weekly until the toxicity resolved, after which hydroxyurea was resumed at the previous dose. If toxicity lasted for more than 2 weeks or recurred after the medication was resumed, the dose was permanently decreased by 2.5 mg/kg/d. Patients were removed from the extension study if they missed more than 3 clinic visits or otherwise failed to comply with the medication regimen.
Statistical analysis
The annual mean value of all hematologic parameters (Hb concentration, MCV, Hb F, F cells, reticulocyte count, WBC count, and platelet count) was calculated and compared with the expected values for untreated patients with Hb SS or Hb Sβ0-thalassemia at the corresponding age, as reported by the Cooperative Study of Sickle Cell Disease (CSSCD).27 The CSSCD was a prospective multicenter investigation of the natural history of sickle cell disease from birth through adulthood that collected data from 23 institutions in a uniform, standardized fashion.28 Children who were younger than 6 months old at enrollment were followed prospectively and made up the “CSSCD infant cohort.”27 Data from the first 5 years of life of this infant cohort (n = 694) were used for comparison with our hydroxyurea-treated patients. The independent t test was used for comparisons within normally distributed data and the Wilcoxon signed rank test for non-normally distributed data.
The observation time was calculated by using the initial 2 years of the original HUSOFT study and adding the follow-up years during the extension study. The incidence rate for VOEs in person-years was compared with those published for age-matched untreated SCA children in the CSSCD29 using the normal-theory test for incidence rate comparisons. The proportion of asplenic patients was compared with the expected proportion among age-matched untreated patients27 using Fisher exact test. All statistical analyses were performed using SAS version 9.1 (SAS Institute, SAS 2002-2003, Cary, NC).
Results
Study enrollment and retention
Twenty-one patients completing 2 years of hydroxyurea treatment in the original HUSOFT trial were eligible to participate in the study extension. The families of all 21 patients agreed to participate in the extension study and signed an additional informed consent in accordance with the Declaration of Helsinki. Twelve patients were female; 20 had Hb SS and 1 had Sβ0-thalassemia; all wereAfrican-American. Their median age at the beginning of the extension study was 3.4 years (range, 2.6 to 4.4 years).
One patient was removed from the study 2 months after dose escalation to 25 mg/kg/d because of noncompliance. The remaining 20 (95%) subjects completed the stepwise dose escalation to 30 mg/kg/d, resulting in a mean (± SD) maximum dose of 30 ± 1.2 mg/kg/d. The only patient not tolerating the dose increase required a permanent dose reduction to 27.5 mg/kg/d because of neutropenia. Splenectomized patients did not differ from nonsplenectomized ones in regard to tolerating drug escalation.
Of the 28 patients enrolled on the original HUSOFT study, 21 completed 2 years, 2 did not complete the study due to noncompliance, the parents of 3 patients elected to discontinue participation, 1 patient had a mild stroke and was placed on chronic transfusion therapy, and 1 died of splenic sequestration.23 Of the 21 patients enrolled on the HUSOFT extension, 17 completed an additional 4 years and 11 were treated with hydroxyurea for a total of 6 years. Reasons for withdrawal from the extension study before 6 years were noncompliance (4 patients), recurrent ACS (1 patient), parent's request to discontinue study drug (4 patients), and death from pneumococcal sepsis (1 patient). The mean duration of hydroxyurea therapy among the 21 patients enrolled in the extension study was 4.9 ± 1.3 years (range, 2.1-6.0 years).
No differences in sex, participating center, age upon study enrollment, enrollment laboratory tests, splenic uptake on the spleen scan, or sickle cell genotype were found among patients completing 6 years of hydroxyurea therapy and those exiting the extension study early (data not shown).
Hematologic efficacy
Mean annual values for Hb, MCV, Hb F, F cells, reticulocyte count, WBC count, and platelet count for years 3 and 4 of hydroxyurea therapy were compared with expected age-matched values for untreated SCA children (Table 1). In hydroxyurea-treated children the mean Hb concentration, MCV, Hb F, and F-cell values were significantly higher, and the mean platelet count, reticulocyte count, and WBC count were significantly lower during years 3 and 4 of extended hydroxyurea therapy. To evaluate the impact of dose escalation, all hematologic indices at the end of year 4 were compared with those at the end of year 2 (Table 2); Hb F values were significantly higher after dose escalation, and other indices did not significantly differ from values at the end of year 2. The mean annual Hb F level for all HUSOFT patients was sustained and often exceeded 20% during extended hydroxyurea therapy (Figure 1), contrasting with the expected decline characteristic of untreated children with SCA.27,30 Splenectomized and nonsplenectomized patients did not differ in their Hb F levels throughout the study.
Toxicity
Mild to moderate neutropenia (ANC 0.5 × 109/L to 1.5 × 109/L) occurred 21 times in 10 patients during year 3 (6.7% of all blood counts performed during year 3) and 21 times in 9 patients during year 4 (9% of all blood counts performed during year 4). Neutropenia was usually associated with a viral illness. Severe neutropenia (ANC less than 0.5 × 109/L) did not occur. Thrombocytopenia was not observed during years 3 and 4 of hydroxyurea therapy but occurred twice during year 5 (platelet counts 36 × 109/L and 68 × 109/L) and once during year 6 (platelet count 68 × 109/L). Thrombocytopenia resolved after temporary dose reduction on both occasions, enabling reescalation of hydroxyurea dosing. Severe anemia was unusual during hydroxyurea treatment, occurring 3 times in 3 patients during year 3, 4 times in 1 patient during year 4, and not at all thereafter. Half of the severe anemia cases were associated with reticulocytopenia, suggesting aplastic crisis as the etiology. Episodes of severe anemia had a mean duration of 5 weeks. No cases of creatinine or ALT elevation were noted, and no cases of myelodysplasia or cancer were observed during this extension study.
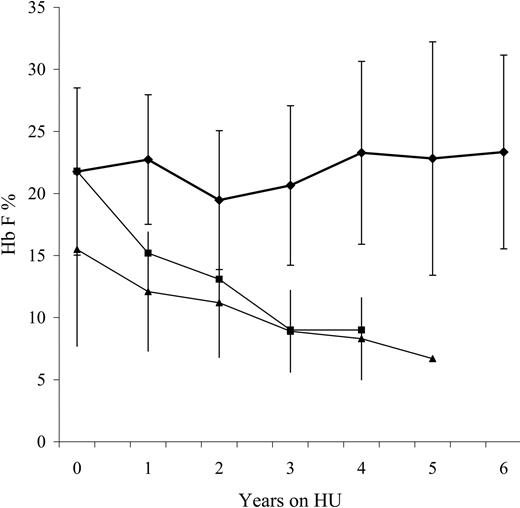
Fetal hemoglobin (Hb F) levels during extended hydroxyurea therapy in infants with SCA. The upper curve (♦) represents the study patients receiving extended hydroxyurea therapy (mean ± SD values for Hb F), while the bottom 2 curves represent the expected values for untreated American27 (▪) and Jamaican children30 (▴) with SCA. Average fetal hemoglobin levels remain close to 20% during extended hydroxyurea therapy.
Fetal hemoglobin (Hb F) levels during extended hydroxyurea therapy in infants with SCA. The upper curve (♦) represents the study patients receiving extended hydroxyurea therapy (mean ± SD values for Hb F), while the bottom 2 curves represent the expected values for untreated American27 (▪) and Jamaican children30 (▴) with SCA. Average fetal hemoglobin levels remain close to 20% during extended hydroxyurea therapy.
One case of acute splenic sequestration occurred during the HUSOFT extension study. Twenty-five months after starting treatment with hydroxyurea, a 32-month-old girl without previous splenomegaly presented with spleen enlargement, a decrease in Hb concentration from 10.5 to 7.9 g/dL (105-79 g/L), and a platelet count drop from 147 × 109/L to 84 × 109/L. She was hospitalized, received one erythrocyte transfusion, and subsequently improved. She did not require splenectomy, and her spleen became nonpalpable a few months after this episode. She remained on hydroxyurea therapy without further problems.
One death occurred during the extension study, caused by sepsis in a 4-year-old girl. This child began hydroxyurea therapy at age 6 months, received 23-polyvalent pneumococcal vaccine (Pneumovax; Merck, White House Station, NJ) at age 2 years, and was judged compliant with both penicillin and hydroxyurea therapy. She had developed an acute episode of splenic sequestration during the original HUSOFT study and underwent elective splenectomy.23 Almost 3.5 years after starting hydroxyurea therapy, and just before the availability of the 7-valent protein-conjugated pneumococcal vaccine (Prevnar; Wyeth Pharmaceuticals, Philadelphia, PA), she developed fever and progressed to cardiovascular collapse within 2 hours. Resuscitation in the emergency department was unsuccessful. Her peripheral blood smear showed diplococci in the neutrophils. Of note, during a routine study visit that occurred only 2 days before this event, she was clinically well and had an ANC of 4.1 × 109/L.
Clinical events
For the entire treatment period, there were 106.4 person-years of observation for this cohort. Thirty-six pain and 8 ACS events were recorded, yielding rates of 33.8 and 7.5 events per 100 person-years, respectively. The expected incidence of pain and ACS for untreated age-matched patients is 32.4 and 24.5 events per 100 person-years, respectively.29 The observed and expected incidence rates were not significantly different for pain events (P = .87). However, unlike the CSSCD study,29 only painful events requiring hospitalization were considered. HUSOFT patients had significantly fewer ACS events (P = .001).
Organ function
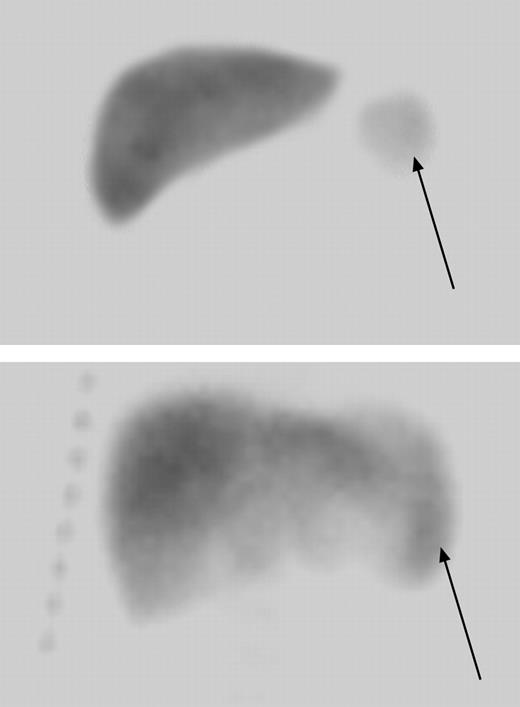
Four patients had undergone surgical splenectomy before enrollment in the original HUSOFT study. There were 17 usplenectomized children enrolled in the extension study, 14 of whom had spleen function assessed at baseline, year 2, and year 4. After 4 years of hydroxyurea therapy, 3 of the 14 (21.5%) children had normal splenic uptake, 3 (21.5%) had decreased uptake, and 2 (14%) had markedly decreased uptake. Only 6 (43%) patients were functionally asplenic (absent uptake) upon study completion, in contrast to the expected 94% incidence of asplenia among untreated age-matched children with SCA based on red-cell pit counts (P < .001).27 Remarkably, one infant with markedly decreased and another with absent splenic uptake prior to hydroxyurea treatment regained normal splenic uptake after 4 years of hydroxyurea therapy (Figure 2). Neither of these 2 patients received erythrocyte transfusions during the extension study.
Fourteen patients had brain MRI and MRA evaluation after 4 years of hydroxyurea therapy. These patients did not differ in age upon study enrollment, sex, disease genotype, and baseline laboratory values with those who did not have brain imaging. Three of the 14 patients (21%) had radiographic evidence of silent infarcts. One girl developed a small focus (less than 0.5 cm) of increased MRI T2-weighted signal in the right periatrial white matter during the study extension. Her transcranial Doppler velocities were normal, and she remained clinically asymptomatic. Another patient with mild stenosis of the proximal right A1 and P2 segments on MRA at year 4 resolved these lesions after 2 more years of hydroxyurea therapy. No radiographic evidence of brain atrophy was observed.
Radionuclide liver-spleen scan showing normalization of splenic uptake after extended hydroxyurea therapy. A 7-month-old child with markedly decreased splenic uptake at baseline (arrow in top panel) regained splenic uptake after 4 years of hydroxyurea therapy (bottom panel). The arrow in the lower panel denotes the location of splenic uptake and indicates a slightly enlarged spleen measuring 9.3 cm in length.
Radionuclide liver-spleen scan showing normalization of splenic uptake after extended hydroxyurea therapy. A 7-month-old child with markedly decreased splenic uptake at baseline (arrow in top panel) regained splenic uptake after 4 years of hydroxyurea therapy (bottom panel). The arrow in the lower panel denotes the location of splenic uptake and indicates a slightly enlarged spleen measuring 9.3 cm in length.
Growth
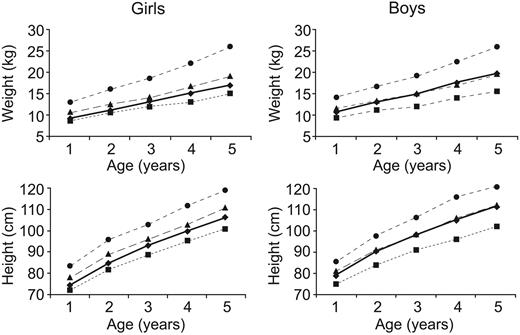
The average weight gain was 2.15 kg per year, height gain 7.9 cm per year, and head circumference increase 1.7 cm per year. These growth rates were within normal limits when plotted on standard pediatric growth curves (Figure 3). Boys (n = 10) demonstrated substantial gains in growth during hydroxyurea treatment, starting therapy at the 25th percentile for weight and 40th percentile for height and reaching the 50th percentile for both weight and height after 4 years of hydroxyurea therapy. The patients treated with hydroxyurea weighed more and were taller than untreated children with Hb SS or Hb Sβ0-thalassemia from 2 to 5 years old as reported by the CSSCD.31 Our patients' growth pattern was similar to that of children with Hb Sβ+-thalassemia and healthy controls.
Discussion
This prospective open-label study demonstrates that prolonged liquid hydroxyurea therapy in very young children with SCA has sustained hematologic efficacy and, in general, limited toxicity. Hydroxyurea dose escalation to 30 mg/kg/d was tolerated in 95% of the children. Hydroxyurea dose escalation to this maximum dose has previously been shown to elicit significantly better hematologic responses in older children than lower doses of 15 to 20 mg/kg/d,13 and among our patients a significant increase in Hb F was observed after dose escalation to 30 mg/kg/d (Table 2). Increased and sustained values of Hb, MCV, Hb F, and F cells were observed during this extension trial, whereas WBC count, platelet count, and reticulocyte values remained lower than expected for untreated children (Table 1). There was a decrease in reticulocyte, WBC, and platelet counts at year 6 compared with year 3, possibly due to the dose escalation. It is unlikely that these decreases will continue during subsequent years of hydroxyurea therapy, based on the recent publication by Zimmerman et al that reported no evidence of cumulative toxicity or marrow exhaustion.13 After escalation to 30 mg/kg/d, continued monitoring of these and other hematologic indices for possible toxicity is advised, however. The sustained response to hydroxyurea suggests good compliance, but adherence to treatment should be prospectively and formally measured in future studies. Taken together, our extended data demonstrate the sustained long-term significant hematologic benefits of hydroxyurea therapy in unselected young children with SCA.
Growth curves for children with sickle cell anemia receiving extended hydroxyurea therapy. The 3rd (▪), 50th (▴), and 97th (•) percentiles for healthy children are illustrated as dotted lines, while the patients (♦) are illustrated as a solid line. Boys increased their average growth rate during hydroxyurea therapy from the 25th percentile for weight and 40th percentile for height to about the 50th percentile for both weight and height after 4 years of hydroxyurea therapy. Girls maintained average growth rates during the treatment period.
Growth curves for children with sickle cell anemia receiving extended hydroxyurea therapy. The 3rd (▪), 50th (▴), and 97th (•) percentiles for healthy children are illustrated as dotted lines, while the patients (♦) are illustrated as a solid line. Boys increased their average growth rate during hydroxyurea therapy from the 25th percentile for weight and 40th percentile for height to about the 50th percentile for both weight and height after 4 years of hydroxyurea therapy. Girls maintained average growth rates during the treatment period.
The average percentage of fetal hemoglobin never declined in treated patients, remaining at approximately 20% from the time of study enrollment (Figure 1), and none of the patients showed decline in Hb F values. Sustained Hb F levels of 20% and above have been associated with reduced clinical events32 and may translate into less organ damage among these infants. However, long-term follow up will be critical to characterize the effect of pharmacologically elevated Hb F in the prevention of organ dysfunction. All patients who tolerated drug dose escalation responded to hydroxyurea by raising the percentage of Hb F, contrasting with studies in adults, in which some patients were unable to sustain a hydroxyurea response.33 Better compliance, higher dosing, higher starting Hb F values, less gene silencing, and/or increased marrow reserve may be related to the superior response rates observed among children, but further investigation is needed.
HUSOFT patients had significantly fewer ACS events than untreated SCA children. This is an important finding due to the morbidity of ACS events. In contrast, however, there was no difference in the incidence of pain, perhaps due to the method of event collection. In the CSSCD study, pain frequency was queried and recorded retrospectively during an annual evaluation; hence, mild events were likely to be forgotten and not always recorded. In HUSOFT, pain was queried and recorded during each monthly visit. It is possible, therefore, that CSSCD relatively underestimated the true pain frequency while HUSOFT relatively overestimated the true pain frequency among young children with SCA.
Hydroxyurea was generally well tolerated, with possibly fewer cases of neutropenia observed in the extension study (8%) than during the first 2 years of therapy (10%), despite the increase in dose. This observation, although not statistically significant, suggests that age may be an important factor for the development of hematologic toxicity, with less myelotoxicity occurring as children become older. The incidence of neutropenia in the study extension approaches the 5.2% rate observed in the HUG-KIDS study, in which school-aged children were treated with hydroxyurea.14 The incidence of sepsis in this group (0.9 cases per 100 patient-years) is within the reported range of 0.0003 to 6.34 cases per 100 person-years among children with SCA on penicillin prophylaxis,34,35 suggesting no increased risk of sepsis among hydroxyurea-treated patients.
We believe hydroxyurea is a relatively safe drug for children with SCA but requires periodic monitoring of blood counts and physical examinations. As with any new treatment, hydroxyurea therapy needs to be closely monitored for toxicity, which may prove difficult in developing countries. Improving the care of SCA patients in underprivileged areas of the world and providing access to new therapies are worthy goals that underscore the need for developing research partnerships among rich and poor countries.36
The possibility of myelodysplasia or malignancy as a long-term side effect of prolonged hydroxyurea use is an ongoing concern. However, a survey of 16 613 patients with sickle cell disease receiving medical care at 52 institutions identified 52 cases of cancer but only 3 patients had any previous exposure to hydroxyurea therapy.37 Among our patients, no cases of cancer developed; however, a longer follow-up is necessary to assess the true incidence of this potential complication. Continued observation should occur for all children with SCA who receive hydroxyurea therapy.
Growth was not impaired among these children treated with hydroxyurea. Using standard growth curves, there was no evidence of growth delay; on the contrary, our patients (especially boys) experienced improved growth, suggesting hydroxyurea may allow growth at rates comparable to those of healthy children and higher than untreated historic controls with SCA. Hydroxyurea has been demonstrated previously to allow weight gain in adult women with SCA,6 to have no adverse effect on growth in school-age children,15 and to promote significantly greater weight and height gain in boys compared with their nontreated counterparts.38 Because the care of sickle cell patients has improved over the past 2 decades, growth comparison with old cohorts has limitations, but more contemporary data are not available for comparison. Hence, prospective studies need to be performed to confirm our findings of possible growth benefit from hydroxyurea.
Possible delay in the progression toward functional asplenia and actual recovery of splenic function in 2 cases were observed during the hydroxyurea extension study. This observation, coupled with the lower proportion of asplenic patients after 4 years of hydroxyurea therapy (43% versus the expected 94%), suggests that hydroxyurea therapy can prevent loss of spleen function or even restore it as reported in older patients.21,22,39 However, the maintenance or recovery of splenic function raises the possibility that these children will be at prolonged risk for acute splenic sequestration. While prospective comparison with untreated patients remains necessary to define if such a risk exists, the management of acute and chronic splenomegaly could emerge as a treatment-associated problem for children with SCA receiving hydroxyurea therapy. Children with prolonged and significant splenomegaly may not tolerate full-dose hydroxyurea therapy14 and may be at long-term risk for acute splenic sequestration. Partial splenectomy could become a therapeutic option for these children, because it offers the advantage of preserving some immune function while controlling acute symptoms of splenomegaly.40,41
Central nervous system abnormalities (ischemic lesions and stenoses) were identified in a few patients, but no cases of brain atrophy were observed. After 4 years of hydroxyurea therapy, the prevalence of silent infarcts was 21% at a median age of 5 years, similar to the prevalence reported among untreated SCA children.42 In one patient stenosis was noted after 4 years of HU therapy but subsequently resolved. Longer follow-up on larger cohorts of young children, coupled with central review of imaging studies, will be needed to determine the risks and benefits of hydroxyurea treatment for the development of cerebrovascular disease in SCA.
In conclusion, the use of oral liquid hydroxyurea at a dose of 30 mg/kg/d in young children with SCA is feasible, results in sustained hematology efficacy with relatively limited adverse events, decreases the ACS rate, and may improve growth. Hydroxyurea possibly decreases the incidence of chronic organ dysfunction such as loss of splenic filtrative function, perhaps by precluding the physiologic decline of fetal hemoglobin. The results of the ongoing National Heart, Lung, and Blood Institute (NHLBI)-sponsored multicenter, randomized, double-blinded clinical trial for very young children with sickle cell anemia (BABY HUG) will further define the role of hydroxyurea in preventing chronic organ damage to the spleen, kidney, and brain.
Appendix
The HUSOFT extension study group members were Evelyn Brown, RN, MSN (Children's Hospital of Wisconsin, Milwaukee, WI); Susan Strawn, RN, and William Schultz, PA-C, MHS (Comprehensive Sickle Cell Center, Saint Jude Children's Research Hospital, Memphis, TN); Juanita Dale, PhD, CPNP, RN (University of Texas, Southwestern Medical Center, Dallas, TX); and Sherri Zimmerman, MD (Duke Pediatric Sickle Cell Program, Division of Hematology-Oncology, Duke University Medical Center, Durham, NC).
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/Blood-2004-12-4973.
A compete list of the members of the Hydroxyurea Safety and Organ Toxicity (HUSOFT) extension study group can be found in the “Appendix.”
Supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and General Clinical Research Center grant nos. M01RR00058 and M01RR00069, National Center for Research Resources, National Institutes of Health.
Presented in part at the 43rd annual meeting of the American Society of Hematology, Orlando, FL, December 10, 2001, and at the 28th annual meeting of the National Sickle Cell Disease Program, Cincinnati, OH, April 13, 2005.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Susan Strawn, RN, William Schultz, PA-C, MHS, Juanita Dale, PhD, CPNP, RN, and Evelyn Brown, RN, for outstanding care offered to our patients; Chin-Shang Li, PhD, and Andy Bush, PhD, for assistance with data analysis; and Julie Groff for help with preparing the figures.