Abstract
Bone marrow cells (BMCs) could correct some pathologic conditions of the central nervous system (CNS) if these cells would effectively repopulate the brain. One such condition is GM1-gangliosidosis, a neurodegenerative glycosphingolipidosis due to deficiency of lysosomal β-galactosidase (β-gal). In this disease, abnormal build up of GM1-ganglioside in the endoplasmic reticulum of brain cells results in calcium imbalance, induction of an unfolded protein response (UPR), and neuronal apoptosis. These processes are accompanied by the activation/proliferation of microglia and the production of inflammatory cytokines. Here we demonstrate that local neuroinflammation promotes the selective activation of chemokines, such as stromal-cell-derived factor 1 (SDF-1), macrophage inflammatory protein 1-α (MIP-1α), and MIP-1β, which chemoattract genetically modified BMCs into the CNS. Mice that underwent bone marrow transplantation showed increased β-gal activity in different brain regions and reduced lysosomal storage. Decreased production of chemokines and effectors of the UPR as well as restoration of neurologic functions accompanied this phenotypic reversion. Our results suggest that β-gal-expressing bone marrow (BM)-derived cells selectively migrate to the CNS under a gradient of chemokines and become a source of correcting enzyme to deficient neurons. Thus, a disease condition such as GM1-gangliosidosis, which is characterized by neurodegeneration and neuroinflammation, may influence the response of the CNS to ex vivo gene therapy.
Introduction
Chemokines comprise a family of about 50 small protein ligands that together with their cognate receptors control leukocyte trafficking during immune surveillance and inflammatory-cell recruitment during host defense.1-3 Besides their known function in the immune system, these molecules have also been implicated in the maintenance of central nervous system (CNS) homeostasis and as a mediator of neuroinflammation.4-8 Two types of interactions primarily control the activity of chemokines in the CNS. The first involves chemokine immobilization by glycosaminoglycans (GAGs), which play a role in the formation of chemokine gradients and trigger localization of leukocyte subpopulations to the site of infection or injury, present at the endothelial surface and the extracellular matrix.9,10 The other interaction entails the tight binding of chemokines to their G-protein-coupled receptors on the surface of leukocytes; this binding activates integrins and promotes their adhesion to the endothelium, followed by their penetration across the endothelial layer into the CNS perivascular space.11-15 In the CNS, chemokines are expressed constitutively at low or negligible levels in astrocytes and microglia, but their expression is rapidly induced by neuroinflammatory conditions16-18 and is mediated by several proinflammatory cytokines (eg, tumor necrosis factor α [TNF-α], interferon γ [IFN-γ], and tumor growth factor β1 [TGF-β1]).19-22 CNS inflammation occurs in several neurodegenerative conditions, including those associated with a lysosomal storage disease (LSD),23,24 and is likely responsible for the recruitment of monocytes and macrophages from peripheral blood. In the murine model of the LSD, GM2-gangliosidosis recruitment of macrophages into the CNS is mediated by the chemokine macrophage inflammatory protein 1-α (MIP-1α) and has been implicated in the pathogenesis of this disease.25
Genetic defects that alter β-galactosidase (β-gal) activity in humans have a devastating effect on the CNS and result in the neurodegenerative LSD GM1-gangliosidosis.26 The clinical phenotypes of this disease demonstrate a continuum of severity: (1) Infantile GM1-gangliosidosis progresses rapidly; developmental arrest occurs soon after birth; and symptoms include profound neurologic deterioration, psychomotor retardation, visceromegaly, and cardiac involvement. Death due to infection or cardiac failure occurs normally within the second year of life. (2) Late-infantile/juvenile GM1-gangliosidosis includes little or no systemic organ involvement, but neurologic problems, ataxia, and seizures develop generally soon after the onset of the symptoms. (3) Chronic GM1-gangliosidosis manifests as slow, progressive dementia, Parkinsonian features, and extrapyramidal signs. At the cellular level, GM1-gangliosidosis affects primarily neurons, which become distended and fill with membranous cytoplasmic bodies, but astrocytes and microglia also appear vacuolated. Abnormal accumulation of GM1-ganglioside (GM1) and to a lesser extent its asialo-derivative, GA1, in neurons is the most prominent biochemical feature of this disease. As with other LSDs, there is no effective cure and only palliative treatment is available.
The mouse model of GM1-gangliosidosis recapitulates the early-onset forms of the disease27 (ie, GM1 and GA1 progressively accumulate in nearly every neuron). Tremor, ataxia, abnormal gait, and gradual deterioration of motor function culminate in the paralysis of the hind limbs.27 Using this model, we recently found that GM1 accumulation in the endoplasmic reticulum (ER) of brain cells and depletion of ER calcium stores stimulate an unfolded protein response (UPR), which in turn, causes neuronal apoptosis.28 The latter process elicits a neuroinflammatory response associated with the activation of inflammatory markers and cytokines, which in turn activate microglia and macrophages at the site of apoptosis.29
Here we demonstrate that neurodegeneration and neuroinflammation in GM1-gangliosidosis mice cause the up-regulation of selected chemokines in specific brain regions. This phenomenon promotes the migration/infiltration of bone marrow cells (BMCs) genetically modified to overexpress β-gal into the CNS, which in turn corrects the neuropathology associated with GM1-gangliosidosis.
Materials and methods
Animals
FVB Glb1+/+ mice (2-6 months) were used as BMC donors, and Glb1-/- mice (3-4 weeks) of the same background were used as recipients.27 All procedures were done in accordance with the US Public Health Service Policy on the Human Care and Use of Laboratory Animals.
Real-time PCR
Stromal-cell-derived factor 1α (SDF-1α) and SDF-1β expression in the mouse was determined by reverse transcription of RNA samples by real-time polymerase chain reaction (PCR). TaqMan Pre-developed Assay Reagent Kits (Applied Biosystems, Foster City, CA) were used to analyze total RNA from cerebellum. Oligotex mRNA Purification Kit (Qiagen, Valencia, CA) was used to analyze polyadenylated (polyA+) RNA from spinal cord. Values were normalized against glyceraldehyde phosphate dehydrogenase (GAPDH) expression, which was amplified simultaneously.
ELISA
SDF-1α protein level was measured by enzyme-linked immunosorbent assay (ELISA) on cerebellar homogenates. Tissue samples were homogenized in antiprotease buffer containing 1 × phosphate-buffered saline (PBS) with Protease Inhibitor Cocktail Tablets (Boehringer Mannheim, Indianapolis, IN). Debris was removed by centrifugation and the aqueous extract stored at -70°C until the time of analyses. Total protein concentration was determined using a bicinchoninic acid reagent (Pierce Chemical, Rockford, IL), and the level of SDF-1α protein was detected using a sandwich-type immunoassay kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
RNAse protection assay
RNA from midbrain, brain stem, and cerebellum were purified and analyzed for β-chemokine expression via RNAse protection assay (BD Biosciences, San Jose, CA) according to the manufacturer's instructions. We used a multiprobe DNA template for the following chemokines: lymphotactin/CXC chemokine ligand 1 (Ltn/CXCL1), regulated upon activation, normal T cells, expressed and secreted (RANTES)/CC chemokine ligand 5 (CCL5), eotaxin/CCL11, MIP-1α/CCL3, MIP-1β/CCL4, MIP-2 CXC ligand 2 (CXCL2), IFN-inducible 10-kDa protein/CXCL10 (IP-10), monocyte chemoattractant protein-1/CCL2 (MCP-1), and T-cell activation antigen (Ag)/CCL1 (TCA-3). L32 and GAPDH served as housekeeping genes. RNAse protection assay (RPA) analysis was performed on 20 μg total RNA and hybridized with probes labeled with [α-32P] uridine triphosphate (UTP). After digestion of ssRNA, the RNA pellet was solubilized and resolved on a 7.5% sequencing gel. Controls included the probe set hybridized to tRNA only, appropriate control RNA, which serves as integrity control for the RNA sample, and a yeast tRNA as a background control. Individual bands were visualized and quantified by a PhosphoImager (Molecular Dynamics, Piscataway, NJ) and analyzed using Image-Quant v1.1 software (Molecular Dynamics).
White blood cell count in cerebrospinal fluid and transmigration assay
Cerebrospinal fluid (CSF) was collected from Glb1+/+ mice and Glb1-/- mice, cytospun onto a slide, and stained with May-Grünwald-Giemsa for total cell counts. CSF was collected from the cisternea magna of the subarachnoid space (fourth ventricle) and peripheral blood by cardiac puncture. For the transmigration assay, leukocytes from peripheral blood were counted on a hemocytometer. Filtered chemotaxis medium containing the CSF (35 μL) was placed in the lower transwell chamber. Blood cells (1 × 106 cells/100 μL) were placed in the upper chamber; chambers were separated by a 3-μm pore filter (Falcon; Becton Dickinson, San Jose, CA). After incubation for 4 hours at 37°C in 5% CO2 atmosphere, the cells that had migrated to the lower chamber were recovered, cytospun onto a microscope slide, stained with May-Grünwald-Giemsa, and counted. Different cell types were distinguished according to their morphologic characteristics and their relative number was calculated.
Vector construction
Transduction of BMCs and BMT
BMCs were isolated from femurs and tibias of Glb1+/+ mice and lysed with Gey solution, and nucleated cells were counted on a hemocytometer. BMCs (2 × 106 cells/mL) were prestimulated in Iscoves medium containing 20 ng/mL mouse interleukin-3 (IL-3), 50 ng/mL human IL-6, and mouse stem-cell factor (R&D Systems) for 48 hours and then transduced with a high titer of MSCV-β-gal-GFP or MSCV-GFP for 48 hours on retronectin-coated plates (Takara, Kyoto, Japan). Transduced cells were analyzed by flow cytometric analysis on a FACScalibur system (Becton Dickinson). Recipient mice (Glb1-/-, Glb1+/+, PPCA-/-) were irradiated (850 cGy) to eliminate endogenous hematopoietic precursors. Genetically modified BMCs (2-6 × 106 cells) overexpressing β-gal and the green fluorescent protein (GFP) marker (MSCV-β-gal-GFP) or only the GFP marker (MSCV-GFP) were then injected into the tail vein of recipients 24 hours after irradiation. For secondary transplants, BMCs were collected from 2 Glb1-/- mice that received a transplant 12 weeks earlier. BMCs (3-9 × 106 cells) collected from these animals were 13% and 35% positive for GFP, and were transplanted into irradiated Glb1-/- mice. Analyses were performed 1, 3, 6, and 9 months after primary and secondary bone marrow transplantations (BMTs).
GFP expression in peripheral-blood samples and β-gal enzyme assay
Blood samples (20 μL) were obtained 1, 3, 6, and 9 months after BMT. Fluorescence activity cell sorting (FACS) analyses of erythrocyte, platelet, and lymphocyte content were done. The level of β-gal activity was measured, as detailed elsewhere.32 The protein concentration was determined using bicinchoninic acid reagent (Pierce Chemical).
Immunohistochemistry
Mouse brains were either formalin-fixed and paraffin-embedded or flash-frozen in liquid nitrogen and then sectioned for immunohistochemistry. The following primary antibodies and dilutions were used: rabbit anti-human β-gal (1:80), rabbit anti-SDF-1β (1:100; Torrey Pines Biolabs, San Diego, CA), rabbit anti-GM1-ganglioside (1:500; US Biological, Swampscott, MA), mouse Alexa Fluor anti-glial fibrillary acidic protein (GFAP) (1:100; Molecular Probes, Eugene, OR), rat anti-F4/80 (1:100; Serotec, Raleigh, NC), rabbit anti-GFP (1:300; Molecular Probes), and mouse anti-NeuN (1:300; Chemicon, Temecula, CA). The following secondary antibodies and dilutions were used: Alexa Fluor 488 and 594 goat antirabbit (1:500; Molecular Probes), fluorescein rabbit antirat (1:100; Vector, Burlingame, CA), biotinylated rabbit antirat (1:100; Vector), biotinylated goat antirabbit (1;300; PharMingen, San Diego, CA), Alexa Fluor rabbit antigoat (1:200; Molecular Probes), Alexa Fluor 594 goat antimouse (1:500; Molecular Probes), and Alexa Fluor 594 rabbit antirat (1:300; Molecular Probes).
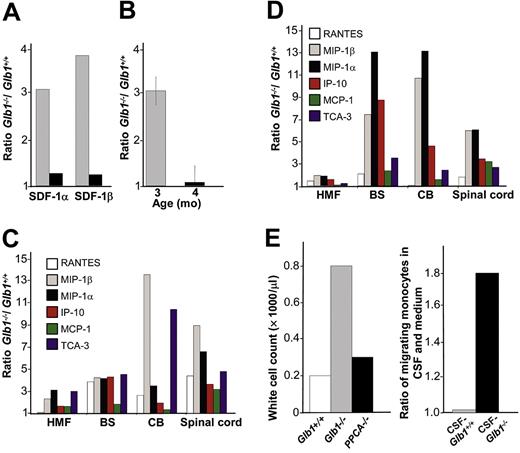
RNA up-regulation of chemokines in the CNS of Glb1-/- mice. (A) Real-time PCR was performed on total RNA extracted from the cerebellum of Glb1+/+ and Glb1-/- mice at 3 months (▦) and 4 months (▪) of age. SDF-1α and SDF-1β mRNAs were higher in Glb1-/- mice than in Glb1+/+ mice. The reactions were standardized to the level of GAPDH mRNA. (B) ELISA of SDF-1α protein in Glb1-/- cerebellum extracts showed that relatively more SDF-1α was expressed at 3 months (▦) than at 4 months (▪) of age. (C-D) RNase protection assay revealed that the β-chemokines RANTES, MIP-1β, MIP-1α, IP-10, MCP-1, and TCA-3 were up-regulated in hindbrain, midbrain, and forebrain regions (HMF); brain stem (BS); cerebellum (CB); and spinal cord of 3- (C) and 4-month-old (D) Glb1-/- mice compared with those of Glb1+/+ controls. The relative levels of RNA induction were normalized against L32 RNA. The amount of chemokines expressed is reported as fold increase over that detected in age-matched Glb1+/+ mice. (E) CSF from 3-month-old Glb1-/- mice contained more white blood cells (WBCs) than did the CSF from PPCA-/- mice or Glb1+/+ littermates. (F) Transmigration assay demonstrated increased monocyte migration toward Glb1-/- CSF than toward Glb1+/+ CSF. Nonspecific cell migration toward serum-free medium was used as a negative control. Data are expressed as mean ± standard deviation (SD).
RNA up-regulation of chemokines in the CNS of Glb1-/- mice. (A) Real-time PCR was performed on total RNA extracted from the cerebellum of Glb1+/+ and Glb1-/- mice at 3 months (▦) and 4 months (▪) of age. SDF-1α and SDF-1β mRNAs were higher in Glb1-/- mice than in Glb1+/+ mice. The reactions were standardized to the level of GAPDH mRNA. (B) ELISA of SDF-1α protein in Glb1-/- cerebellum extracts showed that relatively more SDF-1α was expressed at 3 months (▦) than at 4 months (▪) of age. (C-D) RNase protection assay revealed that the β-chemokines RANTES, MIP-1β, MIP-1α, IP-10, MCP-1, and TCA-3 were up-regulated in hindbrain, midbrain, and forebrain regions (HMF); brain stem (BS); cerebellum (CB); and spinal cord of 3- (C) and 4-month-old (D) Glb1-/- mice compared with those of Glb1+/+ controls. The relative levels of RNA induction were normalized against L32 RNA. The amount of chemokines expressed is reported as fold increase over that detected in age-matched Glb1+/+ mice. (E) CSF from 3-month-old Glb1-/- mice contained more white blood cells (WBCs) than did the CSF from PPCA-/- mice or Glb1+/+ littermates. (F) Transmigration assay demonstrated increased monocyte migration toward Glb1-/- CSF than toward Glb1+/+ CSF. Nonspecific cell migration toward serum-free medium was used as a negative control. Data are expressed as mean ± standard deviation (SD).
Thin-layer chromatography of brain ganglioside fractions
Total lipids were extracted from mouse brain homogenates. The lipid extract corresponding to 100 μg protein was separated on a thin-layer chromatography plate (Silica gel 60 Å; Whatman, Clifton, NJ). Gangliosides were visualized by resorcinol spray and heating. GM1-ganglioside (TRB Pharma, Campinas, Brazil) was used as the standard.
Behavioral testing
Three tests were used to ascertain neurologic function: motor coordination and balance, open-field activity, and walking pattern. Glb1+/+ mice, Glb1-/- mice, and Glb1-/- mice that underwent transplantation were tested during the same session to minimize variability. The ability to maintain balance was tested on a standard rotarod apparatus (Stoelting, Wood Dale, IL).33 Motor and exploratory behaviors were assessed in an acrylic open arena.34 Gait abnormalities were determined by painting the mouse paws with nontoxic, washable paint and then placing the mice in a corridor (10 cm × 10.5 cm × 81.5 cm) lined with white paper.35
Statistical analyses
Data are expressed as mean ± SD and were evaluated by a one-way analysis of variance (ANOVA) followed by the Tukey test for pairwise comparisons. The analyses were performed using the SPSS/PC+ statistical software (SPSS, Chicago, IL) and the mean differences were considered statistically significant when P values were less than .05.
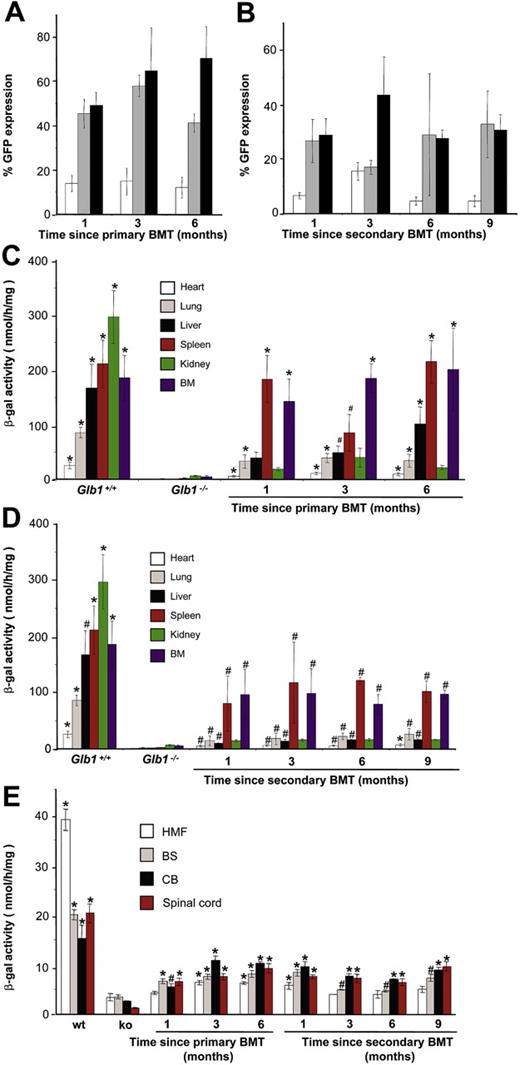
Engraftment of MSCV-β-gal-GFP-transduced BMCs. (A-B) GFP expression in red blood cells (□), platelets (▦), and WBCs (▪) demonstrated consistent, long-term expression of the transgene as long as 6 months after primary transplantation (1 month, n = 7; 3 months, n = 10; 6 months, n = 6) and as long as 9 months after secondary transplantation (1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3). (C-D) Analyses of β-gal activity in systemic tissues of treated mice revealed a significantly higher level of expression of the corrective enzyme after primary (Glb1+/+, n = 7; Glb1-/-, n = 5; 1 month, n = 7; 3 months, n = 8; 6 months, n = 8) and secondary transplantation (Glb1+/+, n = 7; Glb1-/-, n = 3; 1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3) compared with Glb1-/- untreated mice. (E) The β-gal activity was higher in the HMF (hindbrain, midbrain, and forebrain), brain stem, cerebellum, and spinal cord of Glb1-/- mice that underwent primary transplantation and in the brainstem, cerebellum, and spinal cord of Glb1-/- mice that underwent secondary transplantation compared with untreated Glb1-/- mice. Data are expressed as mean ± SD; groups were compared by one-way repeated measures analysis of variance (ANOVA). *P < .001 and #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.
Engraftment of MSCV-β-gal-GFP-transduced BMCs. (A-B) GFP expression in red blood cells (□), platelets (▦), and WBCs (▪) demonstrated consistent, long-term expression of the transgene as long as 6 months after primary transplantation (1 month, n = 7; 3 months, n = 10; 6 months, n = 6) and as long as 9 months after secondary transplantation (1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3). (C-D) Analyses of β-gal activity in systemic tissues of treated mice revealed a significantly higher level of expression of the corrective enzyme after primary (Glb1+/+, n = 7; Glb1-/-, n = 5; 1 month, n = 7; 3 months, n = 8; 6 months, n = 8) and secondary transplantation (Glb1+/+, n = 7; Glb1-/-, n = 3; 1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3) compared with Glb1-/- untreated mice. (E) The β-gal activity was higher in the HMF (hindbrain, midbrain, and forebrain), brain stem, cerebellum, and spinal cord of Glb1-/- mice that underwent primary transplantation and in the brainstem, cerebellum, and spinal cord of Glb1-/- mice that underwent secondary transplantation compared with untreated Glb1-/- mice. Data are expressed as mean ± SD; groups were compared by one-way repeated measures analysis of variance (ANOVA). *P < .001 and #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.
Results
Up-regulation of chemokines in the brains of Glb1-/- mice
We first tested the cytokine-mediated up-regulation of SDF-1, one of the most potent chemoattractant molecules whose expression is induced in several neurodegenerative and neuroinflammatory conditions.36 Real-time PCR and ELISA analyses of Glb1-/- brain regions revealed up-regulation of SDF-1α and SDF-1β isoforms; the highest levels of the SDF-1α and SDF-1β mRNAs (Figure 1A) and SDF-1α protein (Figure 1B) were seen in the cerebellum of 3-month-old mice and, to a lesser extent, 4-month-old mice. Analyses of other chemokines during disease progression demonstrated up-regulation of numerous β-chemokines in specific regions of the brain and spinal cord; their activation also peaked at 3 and 4 months of age. The relative increase in mRNA levels varied depending on the chemokine, the CNS region, and the age of the animal. At 3 months, MIP-1β and TCA-3 had the highest relative expression in the cerebellum, and MIP-1β and MIP-1α were highest in spinal cord (Figure 1C); at 4 months, MIP-1α, MIP-1β, and IP-10 were the highest in brain stem, and MIP-1α and MIP-1β were the highest in the cerebellum and spinal cord (Figure 1D). The level of chemokine expression in PPCA-/- (protective protein/cathepsin A) mice, a model of the LSD galactosialidosis,37 which is associated with storage of glycoproteins rather than glycolipids, was comparable with that of wild-type mice (data not shown).
Increased white blood cell count in the cerebrospinal fluid compartment of Glb1-/- mice
Under normal conditions, some blood-born cells transiently enter the CSF and are released back into the circulation, unless specific stimuli provoke their retention. During CNS inflammation, T cells and monocytes are retained in the subarachnoid space via signals released by the chemokine receptors.38 These migrating cells scavenge invaders and necrotizing tissue debris, thereby helping to repair tissue damage and promote healing.39
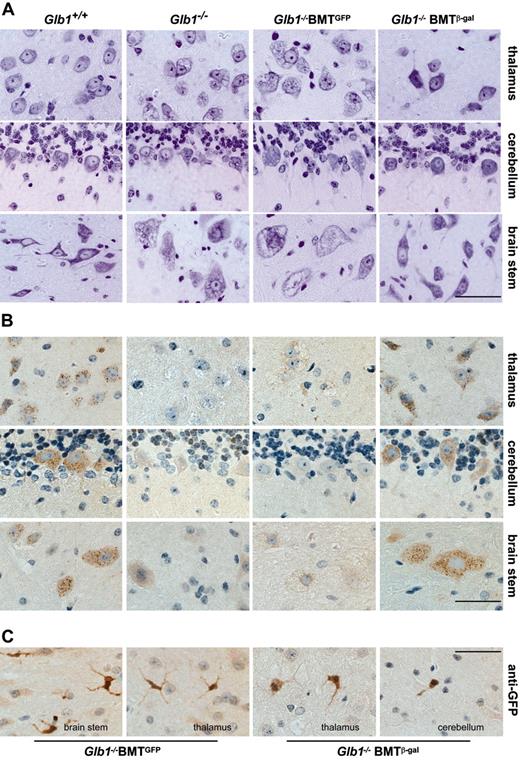
Morphologic analyses in the CNS of treated Glb1-/- mice. (A) Cresyl violet-stained tissue sections of the thalamus, cerebellum, and brain stem from a treated Glb1-/- mouse 3 months after transplantation (Glb1-/- BMT) and from age-matched Glb1+/+ and Glb1-/- mice that underwent mock transplantation (β-gal-/- BMTGFP) revealed restoration of tissue morphology in the treated mouse compared with the extensive vacuolation present in the mice that did not undergo transplantation (Glb1-/-) and the Glb1-/- mice that underwent mock transplantation. (B) Immunolabeling of brain stem, cerebellum, and thalamus revealed the presence of numerous β-gal+ cells in Glb1-/- mice 3 months after BMT. The clear punctate staining demonstrated internalization of the corrective enzyme. Immunolabeling with anti-GFP antibody was done in the same tissues to identify cells of hematopoietic origin. (C) Numerous GFP+ cells in various brain regions showed a ramified microglial morphology. Size bars correspond to 25 μm. Images were visualized using an Olympus BX50 microscope equipped with a 40×/0.65 Plan Apochromatic objective lens (Olympus, Melville, NY) and a Three-Shot 11.3 camera (Diagnostic Instruments, Sterling Heights, MI). Images were acquired with Spot Advanced 4.1.1 software (Diagnostic Instruments) and processed with Adobe Photoshop 8.0 software (Adobe Systems, San Jose, CA).
Morphologic analyses in the CNS of treated Glb1-/- mice. (A) Cresyl violet-stained tissue sections of the thalamus, cerebellum, and brain stem from a treated Glb1-/- mouse 3 months after transplantation (Glb1-/- BMT) and from age-matched Glb1+/+ and Glb1-/- mice that underwent mock transplantation (β-gal-/- BMTGFP) revealed restoration of tissue morphology in the treated mouse compared with the extensive vacuolation present in the mice that did not undergo transplantation (Glb1-/-) and the Glb1-/- mice that underwent mock transplantation. (B) Immunolabeling of brain stem, cerebellum, and thalamus revealed the presence of numerous β-gal+ cells in Glb1-/- mice 3 months after BMT. The clear punctate staining demonstrated internalization of the corrective enzyme. Immunolabeling with anti-GFP antibody was done in the same tissues to identify cells of hematopoietic origin. (C) Numerous GFP+ cells in various brain regions showed a ramified microglial morphology. Size bars correspond to 25 μm. Images were visualized using an Olympus BX50 microscope equipped with a 40×/0.65 Plan Apochromatic objective lens (Olympus, Melville, NY) and a Three-Shot 11.3 camera (Diagnostic Instruments, Sterling Heights, MI). Images were acquired with Spot Advanced 4.1.1 software (Diagnostic Instruments) and processed with Adobe Photoshop 8.0 software (Adobe Systems, San Jose, CA).
At 3 months, the CSF of Glb1-/- mice contained more white blood cells (WBCs) than did that of Glb1+/+ mice. Instead, the number of WBCs in the CSF of PPCA-/- was similar to that of wild-type mice (Figure 1E). Therefore, leukocyte retention in the CSF compartment of Glb1-/- mice is apparently disease specific; this phenomenon usually results from synergistic effects of multiple chemokines.
To verify these results, we performed transmigration assays using CSF from 3-month-old Glb1-/- mice and Glb1+/+ mice as a source of chemoattractants and freshly prepared peripheral WBCs as target cells. The number of WBCs that migrated toward the Glb1-/- CSF was double the number that migrated toward Glb1+/+ CSF (Figure 1F). May-Grünwald-Giemsa staining of migrating cells revealed that most transmigrated cells were monocytes (78%). The number of other WBCs (lymphocytes, 12%; neutrophils, 10%) did not differ substantially between Glb1-/- and Glb1+/+ CSF samples.
Progeny of retrovirally transduced BMCs efficiently migrate into the CNS of Glb1-/- recipients
We used an ex vivo gene therapy approach to investigate whether increased chemokine expression in Glb1-/- mice would facilitate the recruitment of BMCs into the CNS and whether this approach could be a potential treatment for GM1-gangliosidosis. Wild-type BMCs were transduced with either MSCV-β-gal-GFP or MSCV-GFP viral preparations. We first determined that MSCV-β-gal-GFP-transduced BMCs expressed and secreted high levels of the corrective enzyme, which was readily taken up by β-gal-deficient human fibroblasts and restored enzyme activity (Supplemental Figure S1A-B, available at the Blood website; click on the Supplemental Materials link at the top of the online article). For BMT experiments, the transduction efficiency of BMCs with the MSCV-β-gal-GFP vector ranged from 25% to 89%, and the percentage of stem-cell antigen 1-positive (Sca-1+) and cKit+ hematopoietic stem cells (HSCs) expressing GFP and β-gal ranged from 4.15% to 7.64%. The transduction efficiency of BMCs with the MSCV-GFP vector ranged from 18.40% to 67.37%. We used 4 to 6 Glb1-/- recipients for each transplantation in 15 independent experiments. BMCs, transduced with either MSCV-β-gal-GFP or MSCV-GFP (mock transplantations), were transplanted into a total of 52 lethally irradiated 3- to 4-week-old Glb1-/- mice: 43 received MSCV-β-gal-GFP-transduced BMCs and 9 received MSCV-GFP-transduced BMCs. Wild-type (n = 5) and PPCA-/- (n = 3) mice that underwent transplantation were used as BMT controls (Supplemental Table S1). For long-term studies, 11 Glb1-/- mice received a secondary bone marrow (BM) transplant with retrovirally marked BMCs collected from 2 mice that received a primary transplant 3 months earlier. One mouse expressed GFP in 15% of its cells, and the other in 33% of its cells. In both cohorts of treated mice (primary and secondary), GFP-expressing cells of all lineages were detected in peripheral blood samples up to 6 months after primary transplantation (Figure 2A) and up to 9 months after secondary BMT (Figure 2B); this finding indicated efficient engraftment.
GFP expression in peripheral blood was paralleled by an increased level of β-gal activity in most visceral organs (P < .05 relative to untreated mice, post hoc Tukey test). The higher activities were found in the hematopoietic tissues such as the spleen and bone marrow (Figure 2C-D). Immunohistochemical analyses using anti-human β-gal and anti-GFP antibodies identified a substantial number of cells expressing both markers in most of the systemic organs (Supplemental Figure S2). Although Glb1-/- mice showed mild disease in the visceral organs with no overt morphologic changes,27 these data suggested sustained engraftment of BMCs and long-term expression of the transgene.
Transplanted BMCs correct neuropathologic features in Glb1-/- mice
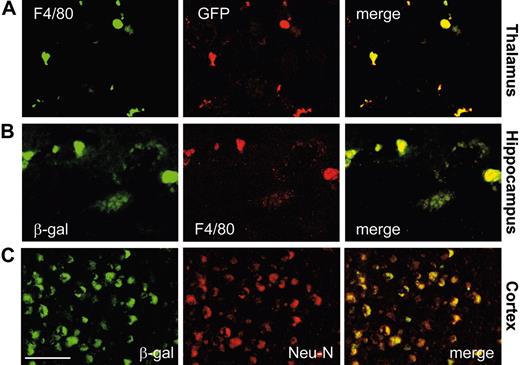
After both primary and secondary BMTs, β-gal activity was significantly higher in the brain and spinal cord of treated Glb1-/- mice than it was in untreated or mock-treated animals (P < .05, post hoc Tukey test) (Figure 2E). The high increment of β-gal activity measured in the cerebellum and brain stem suggested that regions with strong activation of chemokines had preferentially recruited BMCs. We therefore compared the CNS morphology and number of β-gal+ cells in brain and spinal cord sections from animals that received genetically modified MSCV-β-gal-GFP BMCs with those that received MSCV-GFP BMCs (mock). The CNS of Glb1-/- mice that received the therapeutic transgene appeared to have corrected the characteristics associated with the lack of β-gal (ie, lysosomal vacuolation), but in those that underwent mock transplantation the neuropathology persisted (Figure 3A); although the number of GFP+ BM-derived cells was comparable in the 2 groups of mice that underwent transplantation (Figure 3C). The degree of correction varied among treated animals and reflected the percentage of transduced BMCs expressing β-gal that had engrafted and were efficiently recruited to different brain regions. Immunolabeling with anti-β-gal antibody revealed sustained expression of the protein particularly in the thalamus, cerebellar Purkinje cells, and the brain stem (Figure 3B), but also in the cortex, pontine nucleus, medulla oblongata, and hippocampus (Supplemental Figure S3). This preferential and efficient recruitment of BM-derived cells into the brain was not observed in either of the BMT control mice (wild-type and PPCA-/-) that were subjected to the same treatment (data not shown). Many β-gal+ cells also expressed GFP and the microglia marker F4/80 (Figure 4A-B), which confirmed their hematopoietic origin. In fact, GFP-expressing microglia-like cells were identified throughout the brain, particularly in regions with the highest expression of chemokines (ie, cerebellum, brain stem, and thalamus) (Figure 3C). Numerous β-gal-expressing cells were also positive for the neuronal marker NeuN (Figure 4C), a finding that suggested efficient metabolic cooperation between BM-derived cells (ie, monocytes) that migrated into the CNS and the host's neurons. Although not all neurons were devoid of vacuolation, the extent of clearance of lysosomal storage paralleled the percentage of engrafted BMCs overexpressing β-gal. Many neurons displayed the typical punctuated pattern of lysosomes even at the 6- to 9-month time points after BMT (Figure 3B and Supplemental Figure S3). These results confirmed that β-gal-transduced HSCs had successfully engrafted.
Identification of BM-derived vector-expressing cells in the CNS of treated Glb1-/- mice. Immunofluorescence analyses were done on single cryostatic sections from the brains of treated Glb1-/- mice. Fluorescent signals from single sections were sequentially acquired and are shown individually and after merging. (A-B) GFP+ cells and β-gal+ cells were identified as microglia by F4/80 immunoreactivity in Glb1-/- mice 3 months after BMT. (C) Overlay of β-gal staining with the neuronal-specific marker NeuN demonstrated colocalization of the 2 signals, a finding that indicates cross-correction of the enzyme-deficient neurons. Size bar corresponds to 50 μm. Image acquisition was performed as described for Figure 3.
Identification of BM-derived vector-expressing cells in the CNS of treated Glb1-/- mice. Immunofluorescence analyses were done on single cryostatic sections from the brains of treated Glb1-/- mice. Fluorescent signals from single sections were sequentially acquired and are shown individually and after merging. (A-B) GFP+ cells and β-gal+ cells were identified as microglia by F4/80 immunoreactivity in Glb1-/- mice 3 months after BMT. (C) Overlay of β-gal staining with the neuronal-specific marker NeuN demonstrated colocalization of the 2 signals, a finding that indicates cross-correction of the enzyme-deficient neurons. Size bar corresponds to 50 μm. Image acquisition was performed as described for Figure 3.
Enzymatic correction in the CNS reduces GM1 accumulation
To ascertain whether retrovirally transduced BMCs that migrated into the CNS would reverse or retard GM1 accumulation, we measured the amount and distribution of GM1 in different brain regions of the treated mice and compared it with that in age-matched wild-type mice and untreated mice or Glb1-/- mice that underwent mock transplantation (Figure 5). Thin-layer chromatography revealed a clear decrease in the GM1 content in brain stem and cerebellum of treated mice, although the GM1 level in total brain extracts, comprising the hindbrain, midbrain, and forebrain regions, was not substantially different from that in untreated littermates (Figure 5A). The reason for the latter finding was due to the wide heterogeneity of these areas when analyzed together. In fact, immunofluorescence staining of brain sections with anti-GM1 antibody demonstrated a clear reduction of storage in other brain nuclei, such as the hippocampus, of treated Glb1-/- mice compared with mice that underwent mock transplantation (Figure 5B). The decrease in GM1 levels in each brain region correlated with the relative increase in β-gal activity (Figure 5A, bottom).
Ex vivo gene therapy reduces the neuroinflammatory response in Glb1-/- mice
Evidence of successful correction of CNS pathology was further provided by the results of RNAse protection assays, which showed decreased levels of chemokine expression at 3 and 4 months after BMT, a time that corresponded to peak expression of several chemokines in untreated mutant mice (Figure 6A-B). The chemokines that displayed the highest activation in Glb1-/- mice (ie, MIP-1α, MIP-1β, IP-10, and TCA-3) responded best to the treatment. In addition, real-time PCR analyses revealed substantial down-regulation of SDF-1α and SDF-1β mRNAs in the cerebella of treated mice (Figure 6C), and ELISA results revealed an equivalent reduction in SDF-1α protein (Figure 6D).
Attenuation of GM1-ganglioside storage in the brain caused by exogenously delivered β-gal. (A) Thin-layer chromatography of hindbrain, midbrain, and forebrain (HMF), brain stem (BS), and cerebellum (CB) of Glb1+/+, Glb1-/-, and treated Glb1-/- mice 3 months after BMT demonstrated amelioration of GM1-ganglioside storage in the brain stem and cerebellum of the treated mice. The attenuation of GM1-ganglioside accumulation was in agreement with the increment of β-gal activities in those areas (bottom row). GM1-ganglioside was used as a standard. (B) Confocal microscopy with anti-GM1 antibody confirmed less GM1 accumulation in the cerebellum, brain stem, and hippocampus of treated mice (Glb1-/- BMTβ-gal) than in Glb1-/- mice that underwent mock transplantation (Glb1-/- BMTGFP). The arrows indicate cells in which GM1-ganglioside accumulation was most arrested. Size bar corresponds to 50 μm. Image acquisition was performed as described for Figure 3.
Attenuation of GM1-ganglioside storage in the brain caused by exogenously delivered β-gal. (A) Thin-layer chromatography of hindbrain, midbrain, and forebrain (HMF), brain stem (BS), and cerebellum (CB) of Glb1+/+, Glb1-/-, and treated Glb1-/- mice 3 months after BMT demonstrated amelioration of GM1-ganglioside storage in the brain stem and cerebellum of the treated mice. The attenuation of GM1-ganglioside accumulation was in agreement with the increment of β-gal activities in those areas (bottom row). GM1-ganglioside was used as a standard. (B) Confocal microscopy with anti-GM1 antibody confirmed less GM1 accumulation in the cerebellum, brain stem, and hippocampus of treated mice (Glb1-/- BMTβ-gal) than in Glb1-/- mice that underwent mock transplantation (Glb1-/- BMTGFP). The arrows indicate cells in which GM1-ganglioside accumulation was most arrested. Size bar corresponds to 50 μm. Image acquisition was performed as described for Figure 3.
Immunohistochemical labeling with anti-SDF-1β antibody confirmed that considerably fewer SDF-1β+ cells were present in the thalamus of treated Glb1-/- mice than in mutant mice that underwent mock transplantation (Figure 7A). SDF1β-expressing cells were identified as astrocytes by coimmunostaining with anti-GFAP antibody (available as Supplemental Figure S3). Thus, transplantation of retrovirally transduced BMCs expressing the therapeutic enzyme appeared to reverse chemokine activation. The neuroinflammatory response was also reduced, as demonstrated by fewer F4/80+ microglia in the thalamus (shown as example) of treated mice, while Glb1-/- mice that underwent mock transplantation still exhibited numerous spheroid microglia, a morphologic characteristic of their activated status40 (Figure 7A). Using an anti-GFAP antibody, we also found that treated Glb1-/- mice had greatly attenuated astrogliosis particularly in the thalamus (Figure 7A), which is one of the brain nuclei most affected by this phenotypic abnormality in GM1-gangliosidosis mice.29 Overall, these findings indicate decreased neuroinflammation in the treated Glb1-/- mice.
RNA down-regulation of chemokines in brain regions of Glb1-/- mice. (A-B) RNase protection assays revealed that the β-chemokines RANTES, MIP-1β, MIP-1α, IP-10, MCP-1, and TCA-3 were less activated in the hindbrain, midbrain, and forebrain (HMF); brain stem (BS); cerebellum (CB); and spinal cord of treated Glb1-/- mice (-/-BMT) at 3 (A) and 4 (B) months after transplantation than in those regions of untreated Glb1-/- mice (Glb1-/-). The values were normalized to those observed in Glb1+/+ mice. The relative levels of RNA induction were normalized against L32 RNA. (C) At 3 and 4 months of age, the SDF-1α and SDF-1β mRNA levels in the cerebellum of untreated Glb1-/- mice (SDF-1α, ▦; SDF-1β, □) were higher than those in treated Glb1-/- mice (SDF-1α, ▪; SDF-1β, ▧). (D) The amount of SDF-1α protein in the cerebellum was also lower in treated Glb1-/- mice (▪) than it was in untreated Glb1/- mice (▦). Data are expressed as mean ± SD.
RNA down-regulation of chemokines in brain regions of Glb1-/- mice. (A-B) RNase protection assays revealed that the β-chemokines RANTES, MIP-1β, MIP-1α, IP-10, MCP-1, and TCA-3 were less activated in the hindbrain, midbrain, and forebrain (HMF); brain stem (BS); cerebellum (CB); and spinal cord of treated Glb1-/- mice (-/-BMT) at 3 (A) and 4 (B) months after transplantation than in those regions of untreated Glb1-/- mice (Glb1-/-). The values were normalized to those observed in Glb1+/+ mice. The relative levels of RNA induction were normalized against L32 RNA. (C) At 3 and 4 months of age, the SDF-1α and SDF-1β mRNA levels in the cerebellum of untreated Glb1-/- mice (SDF-1α, ▦; SDF-1β, □) were higher than those in treated Glb1-/- mice (SDF-1α, ▪; SDF-1β, ▧). (D) The amount of SDF-1α protein in the cerebellum was also lower in treated Glb1-/- mice (▪) than it was in untreated Glb1/- mice (▦). Data are expressed as mean ± SD.
Ex vivo gene therapy arrests neurodegeneration in Glb1-/- mice
Accumulation of GM1 in the ER of Glb1-/- neurons activates a UPR that ultimately results in neuronal apoptosis mediated by the proapoptotic proteins CHOP (CCAAT/enhancer-binding protein [C/EBP]-homologous protein) and caspase-12.28 We therefore tested whether the decreased GM1 content in the brains of treated Glb1-/- mice would affect the expression of these UPR effectors. Real-time PCR analyses of brain regions and spinal cord of treated Glb1-/- mice showed that the level of CHOP mRNA was practically normalized, and that of caspase-12 was drastically reduced 3 months after transplantation (Figure 7B-C).
Neuromotor abilities progressively worsen in Glb1-/- mice and culminate in ataxia, tremor, and total paralysis of the hind limbs.27 Behavioral testing demonstrated that Glb1-/- mice have an impaired walking pattern: their gait was slow, and they tended to walk in small, labored, uncoordinated movements. Stride strength was significantly reduced, and the paw print length was increased. In contrast, long-term-treated Glb1-/- mice walked faster, with increased stride length and improved coordination. On a rotarod test of balance, the performance of treated Glb1-/- mice was significantly better than that of untreated mutant mice (Figure 7D). Also in an open-field test of locomotor and exploratory activity, treated mice were significantly more active than untreated mutant mice, and the treated mice left the center square of the open field significantly quicker (Figure 7E). In addition, at 6 months after BMT, treated mice showed increased rearing behavior, which indicated improved strength of the hind limbs.
Discussion
The CNS is concealed behind the blood-brain barrier (BBB), which impedes the migration of immune cells and the diffusion of plasma proteins,41,42 giving the CNS an immunologically privileged status. The functional integrity of the BBB is altered in inflammatory diseases of the CNS where leukocytes migrate across the BBB and the brain parenchyma16,43 under control of chemical messengers such as proinflammatory cytokines, chemokines, and adhesion molecules.22,44-46 Proinflammatory cytokines stimulate astrocytes, microglia, and endothelial cells to produce chemokines,47 which in turn activate matrix metalloproteinases that degrade extracellular matrix proteins and disrupt the endothelial tight junctions of the microvessels in the brain.48
Here we provide evidence that the neuroinflammation associated with the neurodegenerative condition in GM1-gangliosidosis mice provokes the up-regulation of selected chemokines in specific brain regions. One of these chemokines is SDF-1, a potent chemoattractant for microglia that has been implicated in controlling the formation of glial scars and the restoration of the BBB after injury.49 SDF-1 participates in the recruitment of mononuclear cells into the CNS, and its intrathecal production is elevated in several neuroinflammatory diseases.50 We have also detected activation of numerous β-chemokines, specifically in the brain stem and cerebellum of Glb1-/- mice. Although the reasons for the time-dependent up-regulation of individual chemokines during the progression of the disease remain unclear, this regulatory pattern may relate to the differential stages of the neuroinflammatory response in mice of different ages. For example, MCP-1 mediates the early response to chemical-induced brain damage,51 and it is the earliest and the most activated chemokine in young Glb1-/- mice. In contrast, MIP-1α, MIP-1β, and IP-10 are produced by glia and infiltrating leukocytes in chronic neurodegenerative diseases19,52,53 ; the activity of these chemokines peaks in older Glb1-/- mice and persists during the later stages of disease. Finally, the number of monocytes in the CSF of Glb1-/- mice was elevated, which is suggestive of chemokine-mediated recruitment and retention of peripheral blood cells in the CSF compartment.
The up-regulation of chemokines in the Glb1-/- mice suggests that this disease condition could create a microenvironment within the CNS that would favor the response to ex vivo gene therapy. Ex vivo gene therapy using virally transduced BMCs expressing the therapeutic enzyme is potentially feasible for treating LSDs, because engrafted BMCs can infiltrate and repopulate organs, including the CNS,54 and cross-correct enzyme-deficient cells. This procedure has been implemented with promising results in other animal models of LSDs.23,55-58 It is becoming increasingly clear, however, that individual LSDs respond differently to the same therapeutic paradigm, most likely because of the nature of the enzyme defect, the accumulated products, and their combined effect on cell metabolism.
Amelioration of inflammatory response, down-regulation of the UPR effectors, and improvement of neuromotor abilities of Glb1-/- mice after BMT. (A) Immunolabeling using an anti-SDF-1β antibody demonstrated that the level of this chemokine was lower in the thalamus of 6-month-old treated Glb1-/- mice (Glb1-/- BMTβ-gal) than in Glb1-/- mice that underwent mock transplantation (Glb1-/- BMTGFP). Anti-GFAP staining demonstrated attenuation of reactive gliosis in the thalamus of 4-month-old treated Glb1-/- mice. Immunolabeling with anti-F4/80 antibody showed that the proliferation and activation of microglia in the thalamus of 3-month-old treated Glb1-/- mice were reverted to a resting state. Size bar corresponds to 50 μm. (B-C) CHOP and caspase-12 mRNA levels in the hindbrain, midbrain, and forebrain (HMF), brain stem (BS), cerebellum (CB), and spinal cord of treated Glb1-/- mice (▪) were restored to levels that were comparable with those seen in wild-type tissues (▦). (D) Glb1-/- mice that underwent transplantation (▪) performed better than untreated Glb1-/- mice (▦) but not as well as wild-type mice (□) on the rotating rod test of motor coordination and balance (n = 15). (E) Similar results were seen on the open-field exploratory activity test (n = 17). Data are expressed as mean ± SD; groups were compared by one-way repeated measures ANOVA. #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.
Amelioration of inflammatory response, down-regulation of the UPR effectors, and improvement of neuromotor abilities of Glb1-/- mice after BMT. (A) Immunolabeling using an anti-SDF-1β antibody demonstrated that the level of this chemokine was lower in the thalamus of 6-month-old treated Glb1-/- mice (Glb1-/- BMTβ-gal) than in Glb1-/- mice that underwent mock transplantation (Glb1-/- BMTGFP). Anti-GFAP staining demonstrated attenuation of reactive gliosis in the thalamus of 4-month-old treated Glb1-/- mice. Immunolabeling with anti-F4/80 antibody showed that the proliferation and activation of microglia in the thalamus of 3-month-old treated Glb1-/- mice were reverted to a resting state. Size bar corresponds to 50 μm. (B-C) CHOP and caspase-12 mRNA levels in the hindbrain, midbrain, and forebrain (HMF), brain stem (BS), cerebellum (CB), and spinal cord of treated Glb1-/- mice (▪) were restored to levels that were comparable with those seen in wild-type tissues (▦). (D) Glb1-/- mice that underwent transplantation (▪) performed better than untreated Glb1-/- mice (▦) but not as well as wild-type mice (□) on the rotating rod test of motor coordination and balance (n = 15). (E) Similar results were seen on the open-field exploratory activity test (n = 17). Data are expressed as mean ± SD; groups were compared by one-way repeated measures ANOVA. #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.
Animals that received primary and secondary transplants of marked BMCs showed persistent GFP expression in peripheral blood and increased enzyme activity in the systemic organs and the CNS. This finding suggests that gene transfer had efficiently targeted long-term repopulating stem cells. The nuclei most affected by GM1-gangliosidosis,27,28 namely the thalamus, pontine nucleus, cerebellum, and brain stem, were also the sites in which chemokines were up-regulated and the neuronal phenotype was most corrected after transplantation. In these areas, we also found a consistent, elevated number of β-gal+ BMCs that also expressed GFP and microglia-specific markers. The restoration of enzyme function in the brain of transplant recipients led to a dramatic decrease in GM1-ganglioside storage and was accompanied by attenuation of the neuroinflammatory response. Most of the disease-activated chemokines, including SDF-1, MIP-1α, MIP-1β, and IP-10, were down-regulated in response to therapy. Remarkably, the levels of transcription of CHOP and caspase-12, 2 components of the UPR pathways responsible for neuronal apoptosis in Glb1-/- mice,28 were also drastically reduced after BMT. Finally, in the brain of treated mice, β-gal-expressing cells persisted long term (up to 9 months after secondary BMT), indicating continuous recruitment of BMCs from circulating precursors. This finding makes it unlikely that radiation-induced tissue damage before BMT was responsible for the recruitment of correcting cells into the CNS.
Normalization of the molecular effectors of CNS pathogenesis was paralleled by a strikingly improved gross appearance of the mice at a time when untreated Glb1-/- animals are severely affected by the disease. Tremor, ataxia, rigidity, and inability to walk and eat improved in animals that underwent BMT 6 to 9 months earlier. Overall performance of treated mice improved on behavioral tests assessing motor function, balance, coordination, and exploratory activity. Together, these results indicate that CNS function was, at least in part, rescued by ex vivo gene therapy. It is important to reiterate, however, that not all mice that received transplants experienced the same extent of correction of their neuronal pathology. Differences in neuronal correction may reflect those in the engraftment of HSCs and in stem-cell-specific proviral integration sites, which may influence the transcriptional activity of the provirus.59,60 Curiously, the BMCs with higher transduction efficiency before transplantation usually engrafted better and persisted longer after transplantation into Glb1-/- mice.
These studies underscore the crucial role of chemokine upregulation in the CNS in response to BMC-mediated therapy of neurodegenerative and neuroinflammatory conditions associated with GM1-gangliosidosis. Since PPCA-/- mice did not show altered levels of chemokines in their CNS, this response seems to be characteristic of only some LSDs and underlines differences in pathogenesis that may ultimately impact the efficacy of therapy. Other glycolipid storage diseases, such as Krabbe disease and GM2-gangliosidosis, also involve up-regulation of these molecular effectors.23-25 In the Twitcher mouse, preferential recruitment of hematopoietic cells to the demyelinating areas of the affected brain is most likely caused by activation of MCP-1 and IL-10; in the GM2-gangliosidosis mouse, time-dependent up-regulation of MIP-1α facilitates the migration of blood cells into the brain parenchyma.25
We observed the most extensive improvement of the disease phenotype in treated Glb1-/- mice at the same time points and brain regions that develop the highest levels of chemokine upregulation, namely at 3 to 4 months after transplantation. We believe that the pattern of activation of chemokines coincides or immediately follows the time-dependent progression of neuronal-cell death and neuroinflammation characteristic of this disease. These results directly implicate chemokines in the recruitment of therapeutic BMCs into the brain in GM1-gangliosidosis and possibly other LSDs. If the latter assumption is correct, the positive response to normal BMT in GM2-gangliosidosis mice and the efficient engraftment of lentivirus-transduced BMCs in the mouse model of metachromatic leukodystrophy can also be attributed to up-regulation of chemokines at specific CNS sites.25,57 Further study of CNS chemokines and leukocyte recruitment into the CNS may lead to improved therapeutic strategies for GM1-gangliosidosis and similar neurodegenerative disorders.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2005-03-1189.
Supported by National Institutes of Health (NIH) grant RO1-DK52025, the Cancer Center Support Grant CA 21765, the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities (ALSAC). A.d'A. holds an Endowed Chair in Genetics and Gene Therapy from the Jewelry Charity Fund; R.S. was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil, and Fundao̧ão Coordenao̧ão de Aperfeio̧oamento de Pessoal de Nível Superior (CAPES), Brazil.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Gerard Grosveld for continuous support; Taylor Walker and Elida Gomero for animal maintenance; William J. Martin for help in the collection of the CSF and blood samples; Huimin Hu for help with the immunohistochemistry; Ann Marie Hamilton-Easton and Richard Ashmun for FACS analyses; Tommaso Nastasi and Erik Bonten for technical suggestions; and Angela McArthur and Charlette Hill for editing and formatting the article. R.S. thanks Roberto Giugliani and Janice Coelho for encouragement and support in preparation of her thesis.